鳖甲煎丸对肝癌Huh-7细胞皮下移植裸鼠模型的影响及作用机制
DOI: 10.12449/JCH260115
Effect and mechanism of Biejiajian Pill on subcutaneous xenograft tumor model of hepatocellular carcinoma Huh7 cells
-
摘要:
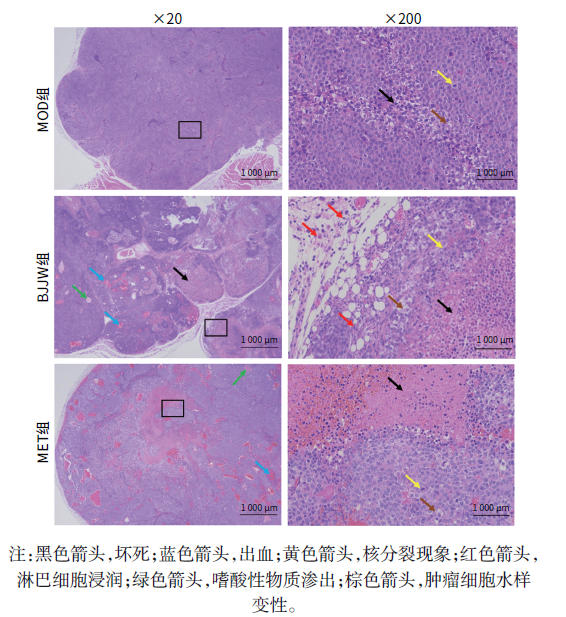
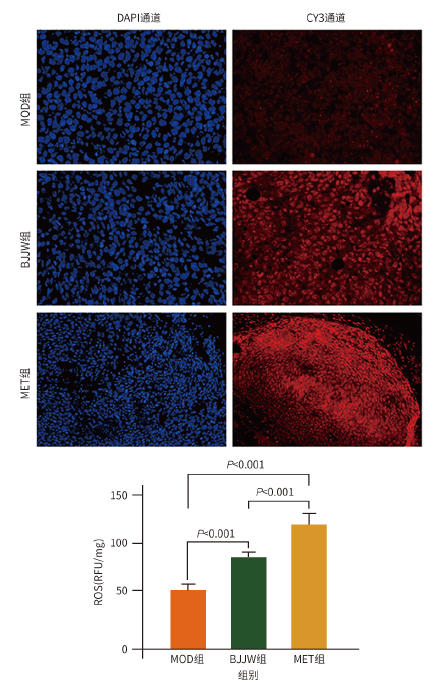
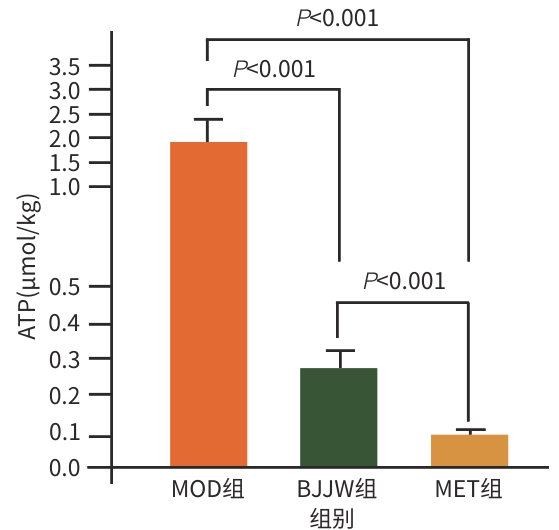
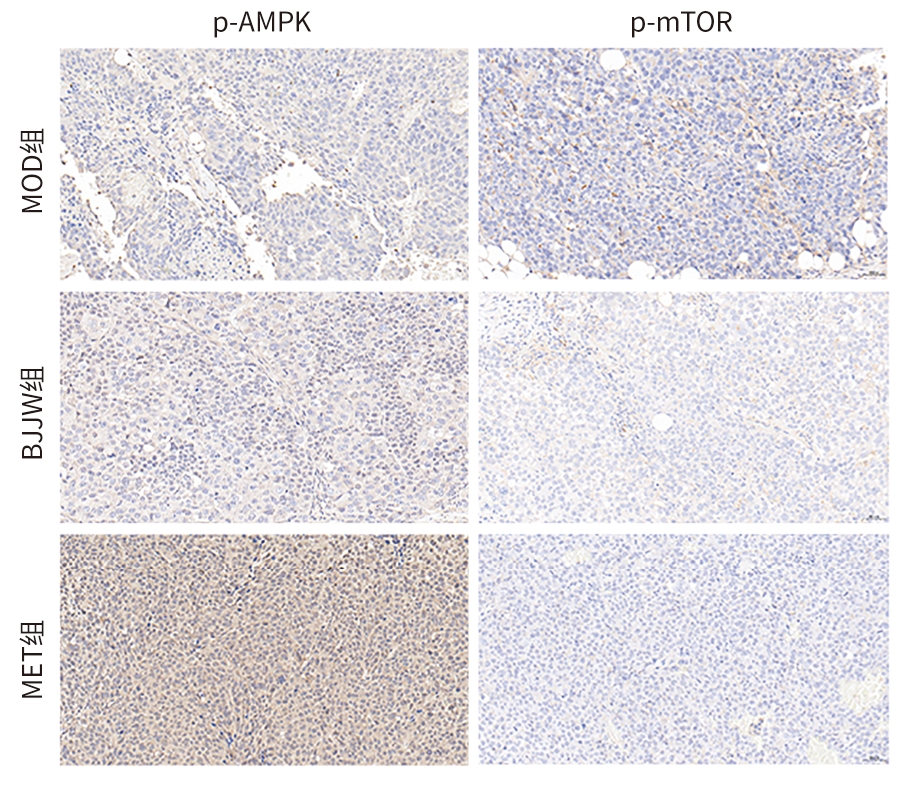
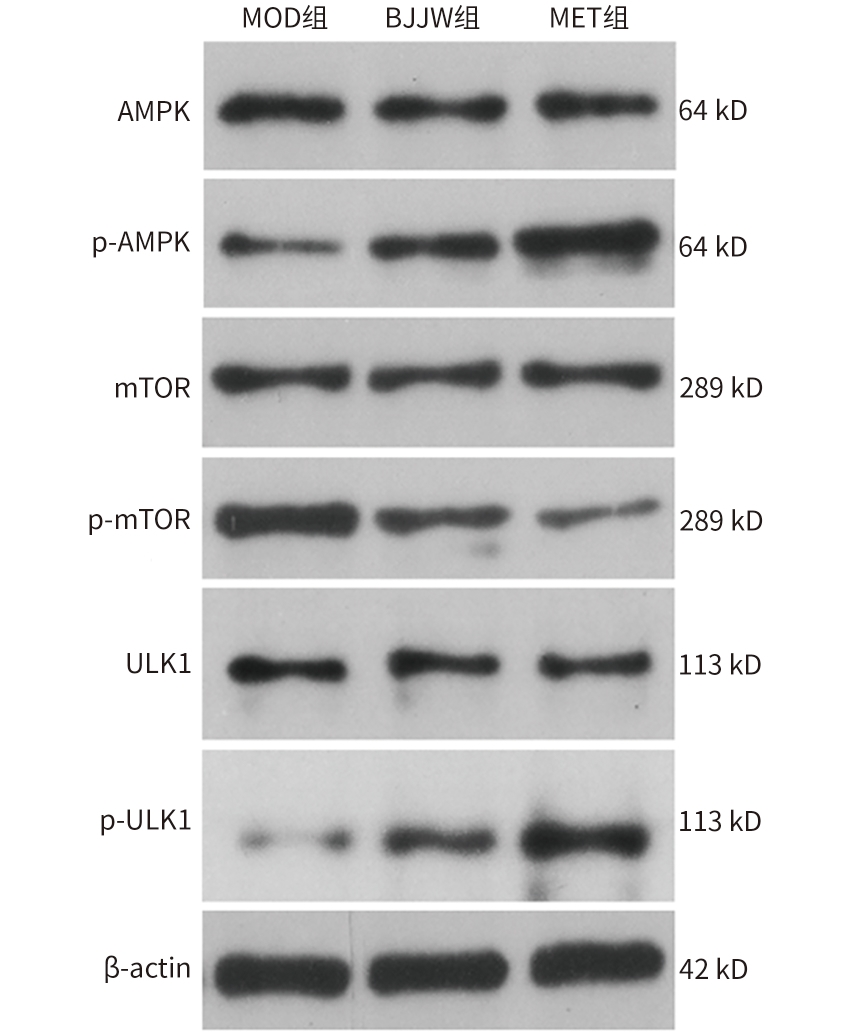
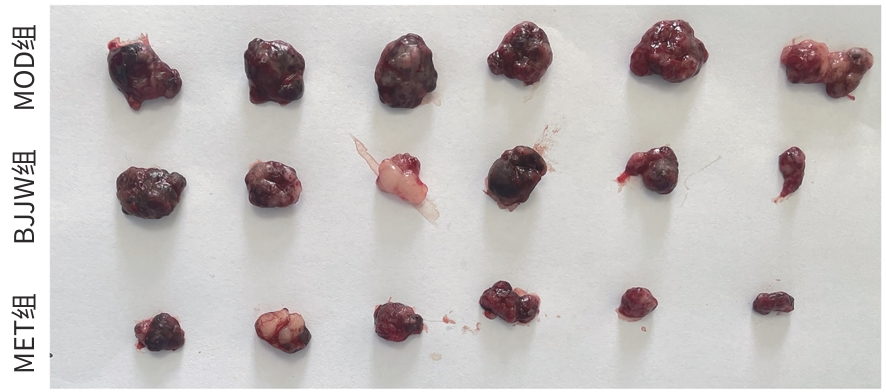
目的 评估鳖甲煎丸对肝癌生长的抑制作用,并阐明其通过线粒体能量代谢介导AMP活化的蛋白质激酶(AMPK)/哺乳动物雷帕霉素靶蛋白(mTOR)通路调控的潜在机制。 方法 采用人肝癌Huh-7细胞建立裸鼠皮下移植瘤模型,将18只荷瘤裸鼠随机分为模型组、鳖甲煎丸组(2.2 g/kg)和二甲双胍组(250 mg/kg),灌胃给药14 d。实验期间监测肿瘤体积与质量;采用HE染色观察组织病理学变化;检测瘤组织活性氧(ROS)与三磷酸腺苷(ATP)水平;采用免疫组化法、蛋白质免疫印迹法检测AMPK/mTOR通路相关蛋白表达水平。满足正态分布的计量资料采用单因素方差分析进行多组间比较,进一步两两比较采用Tukey’s法检验;不满足正态分布的计量资料采用Kruskal-Wallis H检验进行多组间比较,进一步两两比较采用Dunn’s法。 结果 与模型组比较,鳖甲煎丸组相对肿瘤体积与质量均显著降低(P值均<0.01),抑瘤率达45.73%。病理学显示,与模型组比较,鳖甲煎丸组肿瘤细胞数量减少,并出现广泛坏死;机制研究表明,与模型组比较,鳖甲煎丸组ROS水平显著升高(P<0.001),ATP水平显著降低(P<0.001);同时,p-AMPK/AMPK比值(0.81±0.20 vs 0.13±0.04)与p-ULK1/ULK1比值(0.69±0.17 vs 0.18±0.13)均显著升高(P值均<0.01),而p-mTOR/mTOR比值(1.34±0.16 vs 3.20±0.62)显著降低(P<0.01)。 结论 鳖甲煎丸可能通过诱导线粒体能量代谢障碍,即升高ROS水平并降低ATP水平,进而激活AMPK/mTOR信号通路,抑制肝癌生长。 -
关键词:
- 鳖甲煎丸 /
- 肝肿瘤 /
- AMP活化的蛋白质激酶 /
- 哺乳动物雷帕霉素靶蛋白
Abstract:Objective To investigate the inhibitory effect of Biejiajian Pills (BJJW) on the growth of liver cancer, as well as its potential mechanism in mediating the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway through mitochondrial energy metabolism. Methods Human hepatoma Huh7 cells were used to establish a nude mouse model of subcutaneous xenograft tumor. A total of 18 tumor-bearing nude mice were randomly divided into model group, BJJW group (2.2 g/kg), and metformin group (250 mg/kg), and the corresponding drug was given by gavage for 14 consecutive days. Tumor volume and weight were monitored during the experiment; HE staining was used to observe histopathological changes; the levels of reactive oxygen species (ROS) and adenosine triphosphate (ATP) in tumor tissue were measured; immunohistochemistry and Western blotting were used to measure the expression levels of proteins associated with the AMPK/mTOR pathway. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups, and the Tukey’s test was used for further comparison between two groups; the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups, and the Dunn’s test was used for further comparison between two groups. Results Compared with the model group, the BJJW group had a tumor inhibition rate of 45.73%, with significant reductions in both tumor volume and weight (P<0.01). Pathological examination showed that compared with the model group, the BJJW group had a significant reduction in the number of tumor cells and the presence of extensive necrosis. Mechanistic studies showed that compared with the model group, the BJJW group had a significant increase in ROS level (P<0.001) and a significant reduction in ATP level (P<0.001), as well as significant increases in p-AMPK/AMPK ratio (0.81±0.20 vs 0.13±0.04, P<0.01) and p-ULK1/ULK1 ratio (0.69±0.17 vs 0.18±0.13, P<0.01) and a significant reduction in p-mTOR/mTOR ratio (1.34±0.16 vs 3.20±0.62, P<0.01). Conclusion BJJW may inhibit the growth of liver cancer by inducing mitochondrial energy metabolism dysfunction, increasing the level of ROS, reducing the level of ATP, and activating the AMPK/mTOR signaling pathway. -
表 1 鳖甲煎丸对Huh7裸鼠移植瘤体积变化的影响
Table 1. Effect of Biejiajian Pills on hepatocellular subcutaneous tumor mice
组别 动物数
(只)第1天 第3天 第5天 第7天 第9天 第12天 第14天 MOD组 6 106.17±4.19 126.87±8.55 183.98±4.98 291.84±22.53 459.73±83.46 632.40±101.37 829.73±115.17 BJJW组 6 106.61±5.05 117.67±7.66 125.26±12.831) 174.88±28.461) 251.81±85.241)2) 303.09±112.981)2) 408.44±141.951)2) MET组 6 104.47±3.64 124.73±8.78 144.88±18.621)2) 147.09±27.961)2) 180.80±48.031) 222.08±63.491) 272.74±81.031) F值 0.409 1.996 30.004 50.589 22.870 31.401 37.979 P值 0.672 0.170 <0.001 <0.001 <0.001 <0.001 <0.001 注:与MOD组比较,1)P<0.01;与MET组比较,2)P<0.05。
表 2 鳖甲煎丸对Huh7裸鼠移植瘤生长的影响
Table 2. Effect of Biejiajian Pills on the growth of transplanted tumours
组别 动物数(只) RTV 移植瘤质量(g) 肝脏指数(%) 脾脏指数(%) MOD组 6 7.81±1.02 0.61±0.09 40.46±5.42 3.67±0.43 BJJW组 6 3.82±1.351) 0.33±0.1131) 42.77±3.64 3.54±0.27 MET组 6 2.60±0.741) 0.23±0.081) 41.78±2.63 4.15±0.76 F值 39.249 25.342 0.486 2.244 P值 <0.001 <0.001 0.624 0.140 注:与MOD组比较,1)P<0.01。
表 3 鳖甲煎丸对Huh7裸鼠移植瘤组织p-AMPK、p-mTOR蛋白表达水平的影响
Table 3. Effect of Biejiajian Pills on p-AMPK、p-mTOR protein in Huh7 nude mice transplanted tumors
组别 动物数
(只)p-AMPK p-mTOR MOD组 6 2.59(1.57~3.48) 7.74(4.13~13.16) BJJW组 6 7.72(5.54~9.47)1)2) 1.05(0.60~1.67)1)2) MET组 6 17.31(15.69~21.47)1) 0.22(0.14~0.51)1) χ2值 40.48 41.62 P值 <0.001 <0.001 注:与MOD组比较,1)P<0.01;与MET组比较,2)P<0.05。
表 4 鳖甲煎丸对Huh7裸鼠移植瘤组织AMPK/mTOR信号通路相关蛋白表达水平的影响
Table 4. Effect of Biejiajian Pills on expression levels of AMPK/mTOR signaling pathway-related proteins in Huh7 nude mice transplanted tumors
组别 动物数
(只)AMPK p-AMPK p-AMPK/
AMPKmTOR p-mTOR p-mTOR/
mTORULK1 p-ULK1 p-ULK1/
ULK1MOD组 6 0.41±0.03 0.05±0.02 0.13±0.04 0.17±0.02 0.55±0.09 3.20±0.62 0.40±0.10 0.06±0.04 0.18±0.13 BJJW组 6 0.44±0.07 0.36±0.101)2) 0.81±0.201)2) 0.18±0.04 0.24±0.061)2) 1.34±0.161)2) 0.42±0.10 0.28±0.051)2) 0.69±0.171)2) MET组 6 0.35±0.07 0.74±0.131) 2.10±0.091) 0.20±0.04 0.07±0.051) 0.33±0.191) 0.40±0.04 0.71±0.101) 1.79±0.331) F值 4.00 79.41 374.79 0.65 75.88 84.72 0.06 137.75 78.28 P值 0.040 <0.001 <0.001 0.538 <0.001 <0.001 0.943 <0.001 <0.001 注:与MOD组比较,1)P<0.01;与MET组比较,2)P<0.05。
-
[1] DENG XX, LI H, ZHONG YR, et al. Burden of liver cancer attributable to hepatitis B and alcohol globally, in China, and for five sociodemographic index regions from 1990 to 2021: A population-based study[J]. J Clin Transl Hepatol, 2025, 13( 1): 1- 14. DOI: 10.14218/JCTH.2024.00351. [2] ZHOU Y, DING JM, QIN ZY, et al. Predicting the survival rate of patients with hepatocellular carcinoma after thermal ablation by nomograms[J]. Ann Transl Med, 2020, 8( 18): 1159. DOI: 10.21037/atm-20-6116. [3] CAO MD, XIA CF, CAO MM, et al. Attributable liver cancer deaths and disability-adjusted life years in China and worldwide: Profiles and changing trends[J]. Cancer Biol Med, 2024, 21( 8): 679- 691. DOI: 10.20892/j.issn.2095-3941.2024.0149. [4] LIU Y, ZHENG JX, HAO JL, et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019[J]. Cancer Med, 2022, 11( 5): 1310- 1323. DOI: 10.1002/cam4.4551. [5] YU Z, MAN XW, JIANG Y, et al. Analysis of hospitalization expenses and influencing factors of 5 487 patients with liver cancer in Beijing[J]. Chin J Health Stat, 2020, 37( 5): 748- 751. DOI: 10.3969/j.issn.1002-3674.2020.05.029.于哲, 满晓玮, 蒋艳, 等. 北京市5 487例肝癌患者住院费用及影响因素分析[J]. 中国卫生统计, 2020, 37( 5): 748- 751. DOI: 10.3969/j.issn.1002-3674.2020.05.029. [6] RANKOVIĆ B, HAUPTMAN N. Circulating microRNA panels for detection of liver cancers and liver-metastasizing primary cancers[J]. Int J Mol Sci, 2023, 24( 20): 15451. DOI: 10.3390/ijms242015451. [7] ZENG LL, ZHU LT, FU SS, et al. Mitochondrial dysfunction-molecular mechanisms and potential treatment approaches of hepatocellular carcinoma[J]. Mol Cell Biochem, 2025, 480( 4): 2131- 2142. DOI: 10.1007/s11010-024-05144-4. [8] FAUBERT B, SOLMONSON A, DEBERARDINIS RJ. Metabolic reprogramming and cancer progression[J]. Science, 2020, 368( 6487): eaaw5473. DOI: 10.1126/science.aaw5473. [9] LI YF, XU JJ, LAN T, et al. Biejiajian Pill inhibits the malignant biological behavior of Hep3B cells through CMTM6[J]. J Xi’an Jiaotong Univ Med Sci, 2025, 46( 3): 522- 531. DOI: 10.7652/jdyxb202503021.李尹凡, 徐隽婕, 兰涛, 等. 鳖甲煎丸通过CMTM6抑制Hep3B细胞的恶性生物学行为[J]. 西安交通大学学报(医学版), 2025, 46( 3): 522- 531. DOI: 10.7652/jdyxb202503021. [10] SHAO FL, CHEN QP, BI Q, et al. Intervention mechanism of Biejiajian Wan on primary liver cancer by regulating lncRNA SNHG5/miRNA-26a-5p/GSK-3β signal axis[J]. Chin J Exp Tradit Med Formulae, 2024, 30( 4): 107- 113. DOI: 10.13422/j.cnki.syfjx.20230730.邵范雷, 陈秋平, 毕倩, 等. 鳖甲煎丸调控lncRNA SNHG5/miRNA-26a-5p/GSK-3β信号轴干预原发性肝癌的作用机制[J]. 中国实验方剂学杂志, 2024, 30( 4): 107- 113. DOI: 10.13422/j.cnki.syfjx.20230730. [11] FENG MH, HE SQ, HUANG SZ, et al. Inhibitory effect of Biejiajian pills against diethylnitrosamine-induced hepatocarcinogenesis in rats[J]. J South Med Univ, 2020, 40( 8): 1148- 1154. DOI: 10.12122/j.issn.1673-4254.2020.08.12.冯明辉, 贺松其, 黄松泽, 等. 鳖甲煎丸对二乙基亚硝胺诱导的大鼠肝癌的抑制作用及机制[J]. 南方医科大学学报, 2020, 40( 8): 1148- 1154. DOI: 10.12122/j.issn.1673-4254.2020.08.12. [12] YU XP, WANG SY, MENG QG, et al. Exploring regulatory mechanism of Biejiajian pill on hepatocellular carcinoma based on bioinformatics[J]. Chin Arch Tradit Chin Med, 2025, 43( 4): 82- 89. DOI: 10.13193/j.issn.1673-7717.2025.04.015.于欣萍, 王诗尧, 孟庆刚, 等. 基于生物信息学探究鳖甲煎丸对肝细胞癌的调控机制[J]. 中华中医药学刊, 2025, 43( 4): 82- 89. DOI: 10.13193/j.issn.1673-7717.2025.04.015. [13] National Institutes of Health Office of Laboratory Animal Welfare. Animal use and management[EB/OL].( 2024-11-25)[ 2025-04-22]. https://olaw.nih.gov/guidance/topic-index/animal-use.htm. https://olaw.nih.gov/guidance/topic-index/animal-use.htm [14] KUAI Z, CHAO X, HE YT, et al. Metformin attenuates inflammation and boosts autophagy in the liver and intestine of chronologically aged rats[J]. Exp Gerontol, 2023, 184: 112331. DOI: 10.1016/j.exger.2023.112331. [15] WANG GF, GAO H, DAI SN, et al. Metformin inhibits neutrophil extracellular traps-promoted pancreatic carcinogenesis in obese mice[J]. Cancer Lett, 2023, 562: 216155. DOI: 10.1016/j.canlet.2023.216155. [16] YENMIŞ G, BEŞLI N, YAPRAK SARAÇ E, et al. Metformin promotes apoptosis in primary breast cancer cells by downregulation of cyclin D1 and upregulation of P53 through an AMPK-alpha independent mechanism[J]. Turk J Med Sci, 2021, 51( 2): 826- 834. DOI: 10.3906/sag-1908-112. [17] DAS BK, KNOTT RM, GADAD PC. Metformin and asarone inhibit HepG2 cell proliferation in a high glucose environment by regulating AMPK and Akt signaling pathway[J]. Future J Pharm Sci, 2021, 7( 1): 43. DOI: 10.1186/s43094-021-00193-8. [18] LUO S, SCHOOLING CM, WONG ICK, et al. Evaluating the impact of AMPK activation, a target of metformin, on risk of cardiovascular diseases and cancer in the UK Biobank: A Mendelian randomisation study[J]. Diabetologia, 2020, 63( 11): 2349- 2358. DOI: 10.1007/s00125-020-05243-z. [19] TAN QW, XU J, ZHU RH, et al. Effects of Biejia Jianwan on the tumorigenicity and dryness of liver cancer stem cells by regulating miR-140[J]. Jilin J Chin Med, 2024, 44( 1): 90- 95. DOI: 10.13463/j.cnki.jlzyy.2024.01.021.谭钦文, 徐健, 朱荣火, 等. 鳖甲煎丸调控miR-140对肝癌干细胞致瘤性及干性的影响[J]. 吉林中医药, 2024, 44( 1): 90- 95. DOI: 10.13463/j.cnki.jlzyy.2024.01.021. [20] TAN QW, HUANG JJ, ZHONG RX, et al. Effect of Biejia Decoction Pill on aerobic glycolysis in hepatocellular carcinoma by regulating the protein kinase B/mammalian target of rapamycin signaling pathway[J]. J Clin Hepatol, 2025, 41( 2): 300- 306. DOI: 10.12449/JCH250216.谭钦文, 黄晶晶, 钟瑞熙, 等. 鳖甲煎丸调控AKT/mTOR信号通路在肝癌细胞有氧糖酵解中的作用[J]. 临床肝胆病杂志, 2025, 41( 2): 300- 306. DOI: 10.12449/JCH250216. [21] LIN HS, TAN JN, FANG QL, et al. Analysis of the circRNA expression profile in hepatocellular carcinoma cells inhibited by Biejia Jianwan[J]. Genom Appl Biol, 2025, 44( 5): 511- 519. DOI: 10.13417/j.gab.044.000511.林洪升, 谭金娜, 方巧玲, 等. 鳖甲煎丸抑制肝癌细胞的环状RNA表达谱分析[J]. 基因组学与应用生物学, 2025, 44( 5): 511- 519. DOI: 10.13417/j.gab.044.000511. [22] CHEN WG, HE CY, WEN B, et al. Biejiajian pill regulates ferroptosis in hepatocellular carcinoma cells via p62/Keap1/NRF2 signaling pathway: A mechanism study[J]. J Sichuan Univ Med Sci, 2025, 56( 1): 51- 58. DOI: 10.12182/20250160502.陈伟光, 何春雨, 文彬, 等. 鳖甲煎丸通过p62/Keap1/NRF2信号通路调控肝癌细胞铁死亡的作用机制研究[J]. 四川大学学报(医学版), 2025, 56( 1): 51- 58. DOI: 10.12182/20250160502. [23] SUN JL, WEN B, YANG XM, et al. Mechanism research of regulation of proliferation and metastasis of hepatoma cells Hep3B by Biejiajian Pill based on PI3K/AKT/GSK-3β signaling pathway[J]. China J Tradit Chin Med Pharm, 2021, 36( 3): 1361- 1365.孙嘉玲, 文彬, 杨雪梅, 等. 基于PI3K/AKT/GSK-3β信号通路探讨鳖甲煎丸调控肝癌细胞Hep3B增殖转移的机制[J]. 中华中医药杂志, 2021, 36( 3): 1361- 1365. [24] HUANG JJ, HUANG HN, WANG XJ, et al. Bie Jia Jian pill enhances the amelioration of bone mesenchymal stem cells on hepatocellular carcinoma progression[J]. J Nat Med, 2022, 76( 1): 49- 58. DOI: 10.1007/s11418-021-01548-4. [25] WANG WL, SUN Q, WU ZH, et al. Mitochondrial dysfunction-related genes in hepatocellular carcinoma[J]. Front Biosci, 2013, 18( 3): 1141- 1149. DOI: 10.2741/4169. [26] LEE HY, NGA HT, TIAN JW, et al. Mitochondrial metabolic signatures in hepatocellular carcinoma[J]. Cells, 2021, 10( 8): 1901. DOI: 10.3390/cells10081901. [27] van der BLIEK AM, SEDENSKY MM, MORGAN PG. Cell biology of the mitochondrion[J]. Genetics, 2017, 207( 3): 843- 871. DOI: 10.1534/genetics.117.300262. [28] HU CX, HUANG Y, LI LJ. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals[J]. Int J Mol Sci, 2017, 18( 1): 144. DOI: 10.3390/ijms18010144. [29] SHADEL GS, HORVATH TL. Mitochondrial ROS signaling in organismal homeostasis[J]. Cell, 2015, 163( 3): 560- 569. DOI: 10.1016/j.cell.2015.10.001. [30] TIAN CW, LIU YF, LI ZS, et al. Mitochondria related cell death modalities and disease[J]. Front Cell Dev Biol, 2022, 10: 832356. DOI: 10.3389/fcell.2022.832356. [31] HSU CC, PENG DN, CAI Z, et al. AMPK signaling and its targeting in cancer progression and treatment[J]. Semin Cancer Biol, 2022, 85: 52- 68. DOI: 10.1016/j.semcancer.2021.04.006. [32] TREFTS E, SHAW RJ. AMPK: Restoring metabolic homeostasis over space and time[J]. Mol Cell, 2021, 81( 18): 3677- 3690. DOI: 10.1016/j.molcel.2021.08.015. [33] FANG GX, ZHANG PL, LIU JF, et al. Inhibition of GSK-3β activity suppresses HCC malignant phenotype by inhibiting glycolysis via activating AMPK/mTOR signaling[J]. Cancer Lett, 2019, 463: 11- 26. DOI: 10.1016/j.canlet.2019.08.003. [34] LIU YF, XU YY, WANG F, et al. Inhibition of AMPK activity by TRIM11 facilitates cell survival of hepatocellular carcinoma under metabolic stress[J]. Clin Transl Med, 2021, 11( 12): e617. DOI: 10.1002/ctm2.617. [35] MENG SS, GU HW, ZHANG T, et al. Gradual deterioration of fatty liver disease to liver cancer via inhibition of AMPK signaling pathways involved in energy-dependent disorders, cellular aging, and chronic inflammation[J]. Front Oncol, 2023, 13: 1099624. DOI: 10.3389/fonc.2023.1099624. [36] JUNG TY, RYU JE, JANG MM, et al. Naa20, the catalytic subunit of NatB complex, contributes to hepatocellular carcinoma by regulating the LKB1-AMPK-mTOR axis[J]. Exp Mol Med, 2020, 52( 11): 1831- 1844. DOI: 10.1038/s12276-020-00525-3. [37] ZHOU XJ, CHEN Y, WANG FF, et al. Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells[J]. Chem Biol Interact, 2020, 331: 109273. DOI: 10.1016/j.cbi.2020.109273. [38] JIA L, LIN XR, GUO WY, et al. Salvia chinensia Benth induces autophagy in esophageal cancer cells via AMPK/ULK1 signaling pathway[J]. Front Pharmacol, 2022, 13: 995344. DOI: 10.3389/fphar.2022.995344. [39] NAIK PP, MUKHOPADHYAY S, PRAHARAJ PP, et al. Secretory clusterin promotes oral cancer cell survival via inhibiting apoptosis by activation of autophagy in AMPK/mTOR/ULK1 dependent pathway[J]. Life Sci, 2021, 264: 118722. DOI: 10.1016/j.lfs.2020.118722. [40] WANG CM, CIGLIANO A, JIANG LJ, et al. 4EBP1/eIF4E and p70S6K/RPS6 axes play critical and distinct roles in hepatocarcinogenesis driven by AKT and N-Ras proto-oncogenes in mice[J]. Hepatology, 2015, 61( 1): 200- 213. DOI: 10.1002/hep.27396. [41] XU M, WANG ZJ, WU ZS, et al. Autophagy activated by the AMPK/mTOR/ULK1 pathway involves AURKB-mediated microgliosis in neuropathic pain[J]. Brain Behav Immun, 2025, 129: 948- 959. DOI: 10.1016/j.bbi.2025.07.019. -



 PDF下载 ( 24411 KB)
PDF下载 ( 24411 KB)

 下载:
下载: