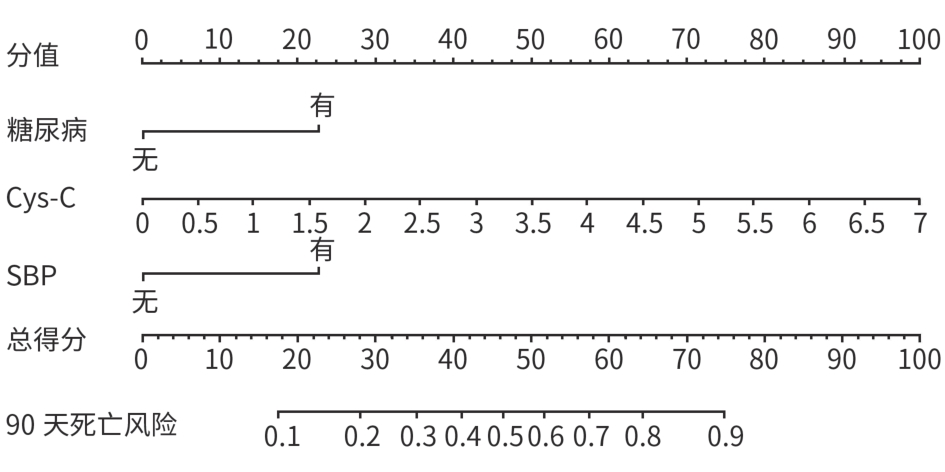
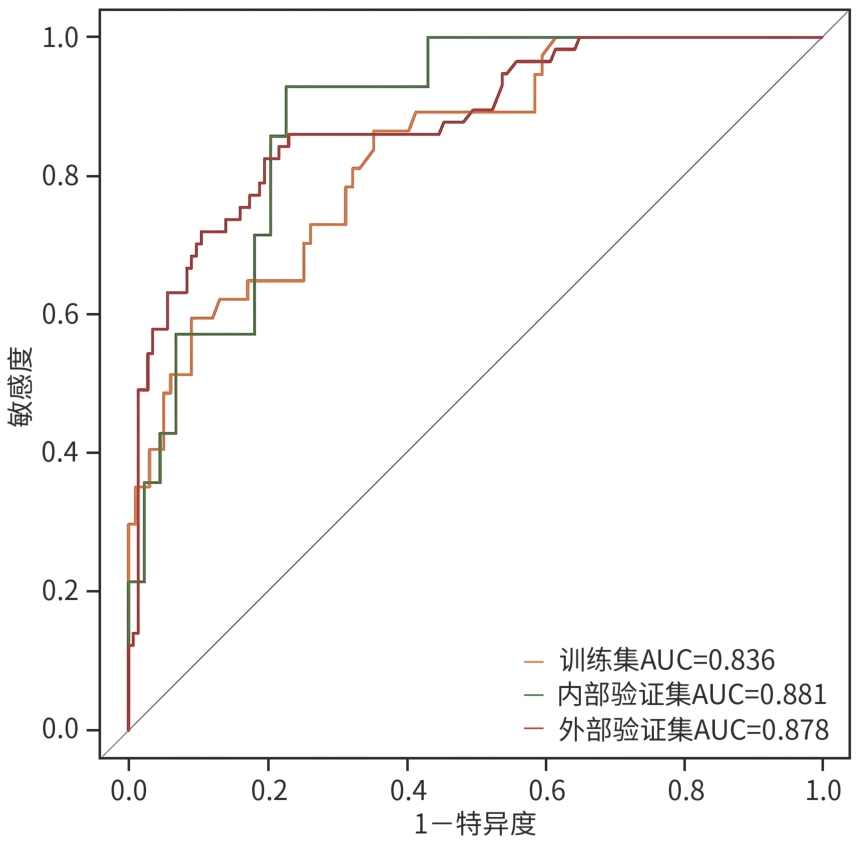
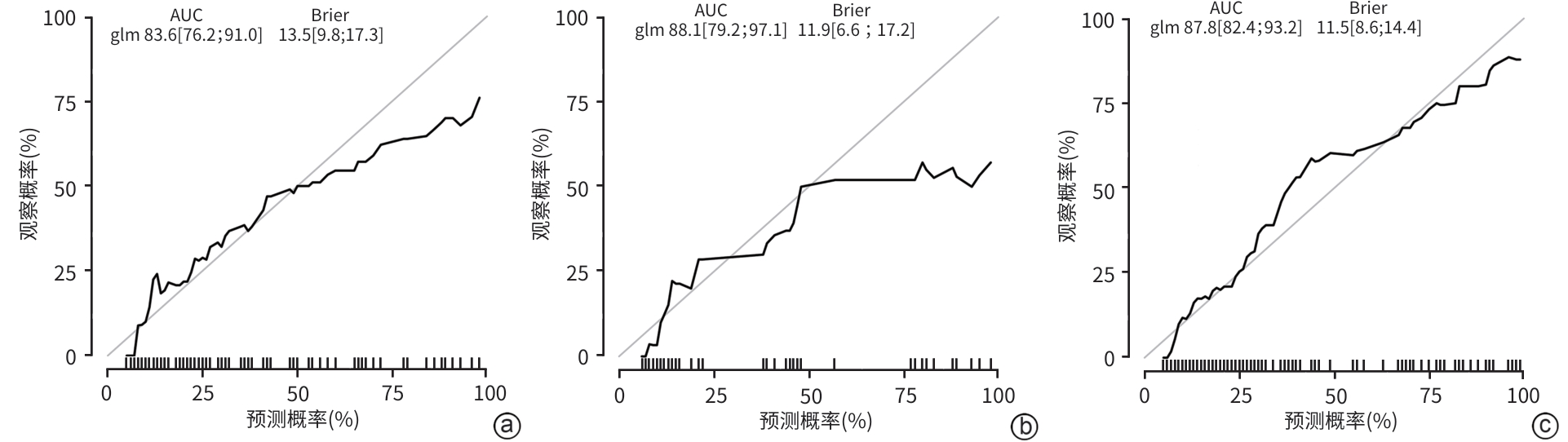
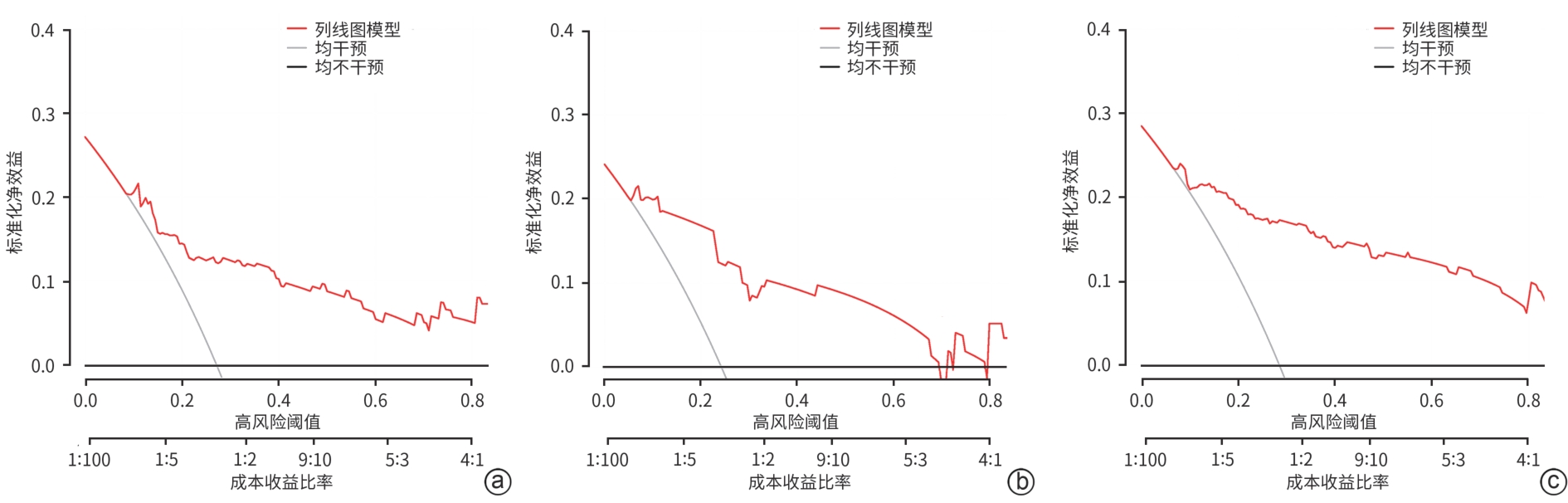
慢加急性肝衰竭患者90天死亡的危险因素分析及预测模型构建
DOI: 10.12449/JCH260118
Risk factors for 90-day mortality in patients with acute-on-chronic liver failure and establishment of a predictive model
-
摘要:
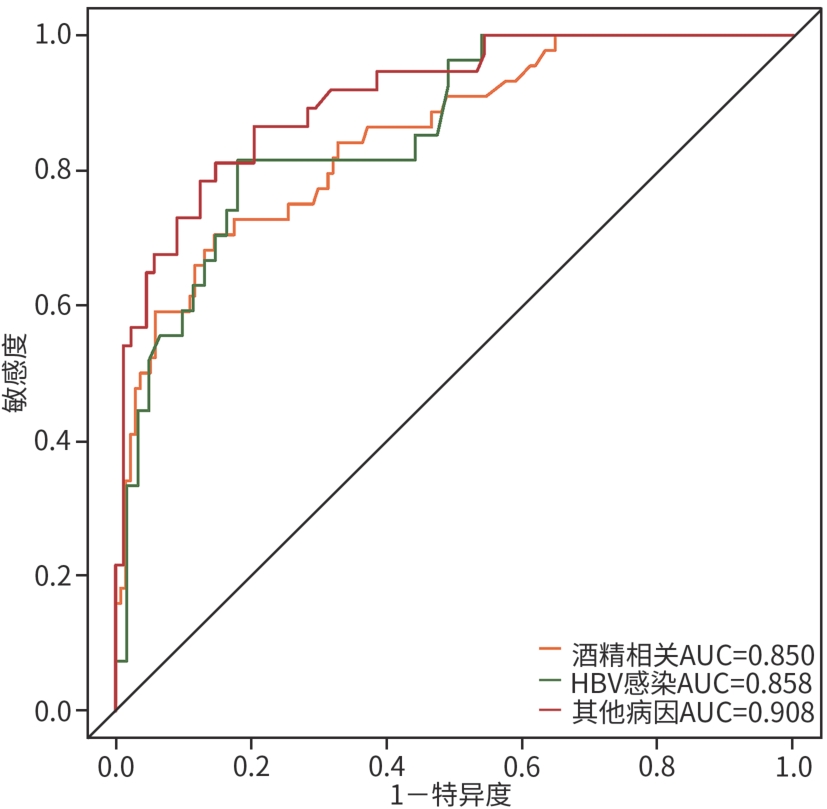
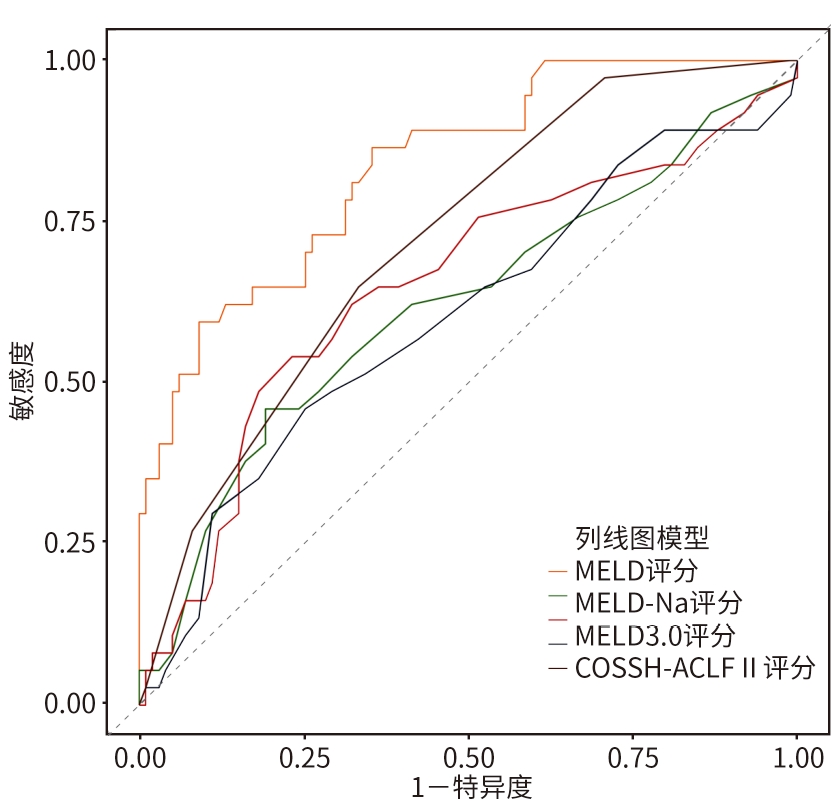
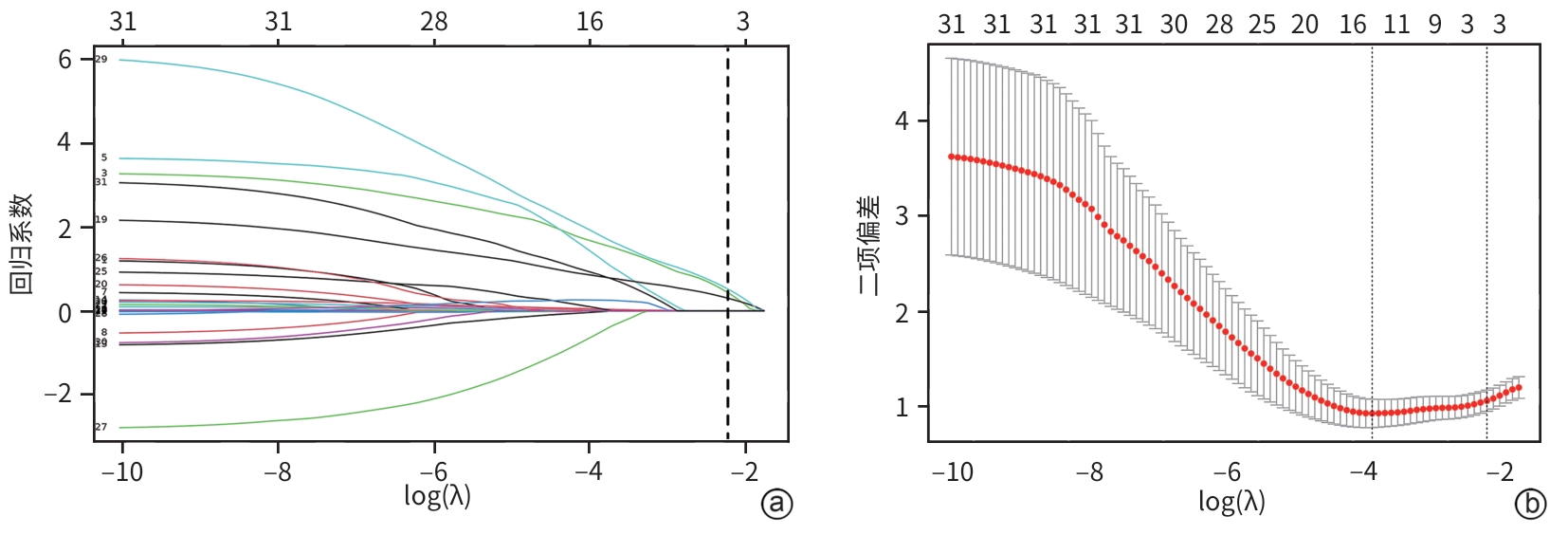
目的 本研究旨在探讨影响慢加急性肝衰竭(ACLF)患者90天死亡的独立预测因子,并构建风险预测模型,评估其与终末期肝病模型(MELD)、MELD-Na、MELD 3.0和中国重症乙型肝炎研究小组慢加急性肝衰竭2.0(COSSH-ACLF Ⅱ)评分的预测效能差异。 方法 回顾性分析2018年7月—2024年7月内蒙古医科大学附属医院和呼和浩特市第二医院收治的394例ACLF患者临床资料。收集患者一般资料及入院时实验室指标,定量资料两组间比较采用成组t检验或Mann-Whitney U检验,定性资料两组间比较采用χ2检验或校正χ2检验。采用LASSO回归筛选变量,多因素Logistic回归构建预测模型并绘制列线图。通过受试者操作特征曲线(ROC曲线)及曲线下面积(AUC)、校准曲线和临床决策曲线评估模型性能。 结果 本研究共纳入394例ACLF患者,其中训练集和内部验证集分别为136例和58例,外部验证集200例。全队列年龄(52.9±11.7)岁,男性占72.84%(287/394),HBV感染者占22.33%(88/394),酒精相关占45.94%(181/394),其他原因(包括药物性、自身免疫性等)占31.73%(125/394)。90天总体病死率为27.41%(108/394)。多因素Logistic回归显示,糖尿病[比值比(OR)=5.831, 95%置信区间(CI): 1.587~21.424, P=0.008]、胱抑素-C(Cys-C)(OR=2.984, 95%CI: 1.501~5.933, P=0.002)和自发性细菌性腹膜炎(SBP)(OR=5.692, 95%CI: 2.150~15.071, P<0.001)均为独立危险因素,并以此绘制列线图。模型在训练集、内部验证集和外部验证集的AUC分别为0.836、0.881、0.878,显示出良好的区分度。校准曲线拟合良好,并有较高的临床净获益。按病因进行亚组分析显示,模型在HBV感染、酒精及其他病因所致ACLF患者中的AUC分别为0.850、0.858和0.908,表明该模型在不同病因人群中均具有良好的区分能力。与传统评分相比,该模型(AUC=0.836)显著优于单独使用MELD(AUC=0.619,Z=3.197,P=0.001)、MELD-Na(AUC=0.651,Z=2.998,P=0.003)、MELD 3.0(AUC=0.601, Z=3.682,P<0.001)和COSSH-ACLFⅡ(AUC=0.719, Z=2.396,P=0.017)评分的预测价值。 结论 糖尿病、SBP和Cys-C是ACLF患者90天死亡的独立危险因素。与MELD、MELD-Na、MELD 3.0和COSSH-ACLFⅡ评分相比,该模型对ACLF患者90天预后具有更高的预测价值,且适用于多种病因所致的ACLF患者。 Abstract:Objective To investigate the independent predictive factors for 90-day mortality in patients with acute-on-chronic liver failure (ACLF), to establish a risk predictive model, and to assess its predictive efficacy in comparison with MELD, MELD-Na, MELD 3.0, and COSSH-ACLF Ⅱ. Methods A retrospective analysis was performed for the clinical data of 394 patients with ACLF who were admitted to The Affiliated Hospital of Inner Mongolia Medical University and Hohhot Second Hospital from July 2018 to July 2024, and general information and laboratory markers on admission were collected from all patients. The independent-samples t test or the Mann-Whitney U test was used for comparison of quantitative data between two groups, and the chi-square test or the adjusted chi-square test was used for comparison of qualitative data between two groups. The LASSO regression analysis was used to identify related variables, and the multivariate logistic regression analysis was used to establish a predictive model and generate a nomogram. The receiver operating characteristic (ROC) curve, the area under the ROC curve (AUC), calibration curve, and clinical decision curve were used to assess the performance of the model. Results A total of 394 patients with ACLF were included in this study, with 136 patients in the training set, 58 in the internal validation set, and 200 in the external validation set. The cohort had a mean age of 52.9±11.7 years, among whom male patients accounted for 72.84% (287/394), the patients with HBV infection accounted for 22.33% (88/394), the patients with alcohol-related causes accounted for 45.94% (181/394), and the patients with other causes (including drug-induced and autoimmune diseases) accounted for 31.73% (125/394). The overall 90-day mortality rate was 27.41% (108/394). The multivariate logistic regression analysis showed that diabetes (odds ratio [OR]= 5.831, 95% confidence interval [CI]: 1.587 — 21.424, P=0.008), cystatin C (Cys-C) (OR=2.984, 95%CI: 1.501 — 5.933, P=0.002), and spontaneous peritonitis (SBP) (OR=5.692, 95%CI: 2.150 — 15.071, P<0.001) were independent risk factors, and a nomogram was generated based on these factors. This model had an AUC of 0.836 in the training set, 0.881 in the internal validation set, and 0.878 in the external validation set, showing a good discriminatory ability. The calibration curve showed a good degree of fitting, with a relatively high net clinical benefit. The subgroup analysis based on etiology showed that the model had an AUC of 0.850 in the patients with HBV infection, 0.858 in the patients with alcohol-induced ACLF, and 0.908 in the patients with other etiologies, indicating that the model had a good discriminatory ability across the populations with different etiologies. Compared with traditional scores, the model (AUC=0.836) had a significantly better predictive value than MELD (AUC=0.619, Z=3.197, P=0.001), MELD-Na (AUC=0.651, Z=2.998, P=0.003), MELD 3.0 (AUC=0.601, Z=3.682, P<0.001), and COSSH-ACLF Ⅱ (AUC=0.719, Z=2.396, P=0.017) alone. Conclusion Diabetes, SBP, and Cys-C are independent risk factors for 90-day mortality in patients with ACLF. Compared with MELD, MELD-Na, MELD 3.0, and COSSH-ACLF Ⅱ scores, this model has a higher predictive value for 90-day prognosis in patients with ACLF and is suitable for patients with ACLF caused by various etiologies. -
Key words:
- Acute-on-Chronic Liver Failure /
- Prognosis /
- Risk Factors /
- Nomogram
-
表 1 训练集和内部验证集基线特征比较
Table 1. Comparison of baseline characteristics between the training set and the internal validation
指标 训练集(n=136) 内部验证集(n=58) 统计值 P值 年龄(岁) 52.45±11.77 52.66±10.49 t=-0.12 0.908 男/女(例) 96/40 48/10 χ²=3.15 0.076 病因[例(%)] 酒精相关 73(53.68) 35(60.34) χ²=0.73 0.392 HBV感染 17(12.50) 9(15.52) χ²=0.32 0.572 其他 46(33.82) 14(24.14) χ²=1.79 0.181 糖尿病[例(%)] 16(11.76) 8(13.79) χ²=0.15 0.694 高血压[例(%)] 26(19.12) 6(10.34) χ²=2.27 0.132 肝硬化[例(%)] 116(85.29) 48(82.76) χ²=0.20 0.655 腹水[例(%)] 99(72.79) 47(81.03) χ²=1.48 0.223 HE [例(%)] 54(39.71) 18(31.03) χ²=1.31 0.252 SBP[例(%)] 32(23.53) 17(29.31) χ²=0.72 0.396 WBC(×109/L) 7.04(4.48~9.84) 5.96(4.27~9.14) Z=-1.18 0.237 NEU(×109/L) 5.17(2.96~7.96) 4.19(2.70~7.30) Z=-0.86 0.391 Hb(g/L) 110.00(88.00~127.75) 107.50(90.75~122.00) Z=-0.38 0.701 PLT(×109/L) 77.00(53.00~107.00) 74.50(51.75~114.50) Z=-0.23 0.821 INR 1.84(1.63~2.16) 1.77(1.61~1.98) Z=-1.31 0.189 PTA(%) 35.10(28.25~41.20) 35.65(32.00~41.70) Z=-1.03 0.303 ALT(U/L) 37.80(24.43~67.43) 42.60(22.83~82.00) Z=-0.30 0.763 AST(U/L) 80.00(48.85~127.30) 89.50(53.75~148.08) Z=-0.95 0.342 PAB(mg/dL) 3.70(2.20~5.58) 5.15(2.75~6.85) Z=1.93 0.053 Alb(g/L) 26.35(23.03~29.98) 27.20(23.28~29.45) Z=-0.51 0.613 GLO(g/L) 33.32±10.44 33.51±9.05 t=-0.12 0.906 TBil(μmol/L) 197.00(123.48~383.30) 180.20(124.13~280.95) Z=-0.73 0.466 Ur(mmol/L) 6.24(3.90~10.10) 5.15(3.40~7.85) Z=-1.48 0.138 Cr(μmol/L) 68.50(52.00~93.75) 67.50(51.75~100.25) Z=-0.12 0.908 Cys-C(mg/L) 1.35(1.03~1.76) 1.30(1.00~1.90) Z=-0.08 0.939 K+(mmol/L) 3.70(3.25~4.20) 3.68(3.10~4.17) Z=-0.55 0.584 Na+(mmol/L) 135.00(132.00~137.50) 134.00(131.00~137.00) Z=-0.86 0.392 GLU(mmol/L) 5.61(4.71~7.28) 5.65(5.08~6.75) Z=-0.89 0.375 TC(mmol/L) 2.11(1.54~2.88) 2.28(1.71~3.06) Z=-1.44 0.149 TG(mmol/L) 0.80(0.61~1.35) 0.77(0.56~1.23) Z=-1.18 0.238 HDL-C(mmol/L) 0.22(0.11~0.45) 0.32(0.15~0.48) Z=-1.58 0.115 MELD评分(分) 20.00(16.00~25.75) 20.00(16.00~24.00) Z=-0.52 0.604 MELD-Na评分(分) 22.00(18.00~29.00) 23.00(19.00~27.25) Z=-0.11 0.914 MELD 3.0评分(分) 26.00(23.00~30.00) 25.00(23.00~29.00) Z=-0.51 0.612 COSSH-ACLFⅡ评分(分) 7.00(7.00~8.00) 7.00(6.00~8.00) Z=-1.15 0.251 90天死亡 [例(%)] 37(27.21) 14(24.14) χ²=0.20 0.657 注:HBV,乙型肝炎病毒;HE,肝性脑病;SBP,自发性细菌性腹膜炎;WBC,白细胞计数;NEU,中性粒细胞计数;Hb,血红蛋白;PLT,血小板计数;INR,国际标准化比值;PTA,凝血酶原活动度;ALT,丙氨酸氨基转移酶;AST,天冬氨酸氨基转移酶;PAB,前白蛋白;Alb,白蛋白;GLO,球蛋白;TBil,总胆红素;Ur,尿素;Cr,肌酐;Cys-C,胱抑素-C;GLU,血糖;TC,总胆固醇;TG,甘油三酯;HDL-C,高密度脂蛋白胆固醇。
表 2 训练集基线特征比较
Table 2. Comparison of baseline characteristics within the training set
指标 生存组(n=99) 死亡组(n=37) 统计值 P值 年龄(岁) 50.74±11.99 57.03±9.93 t=-2.85 0.005 男/女(例) 74/25 22/15 χ²=3.03 0.082 病因[例(%)] 酒精相关 57(57.58) 16(43.23) χ²=2.23 0.136 HBV感染 11(11.11) 6(16.22) χ²=0.26 0.610 其他 31(31.31) 15(40.54) χ²=1.03 0.311 糖尿病[例(%)] 5(5.05) 11(29.73) χ²=13.51 <0.001 高血压[例(%)] 19(19.19) 7(18.92) χ²=0.00 0.971 肝硬化[例(%)] 81(81.82) 35(94.59) χ²=3.51 0.061 腹水[例(%)] 73(73.74) 26(70.27) χ²=0.16 0.686 HE [例(%)] 36(36.36) 18(48.65) χ²=1.70 0.193 SBP[例(%)] 14(14.14) 18(48.65) χ²=17.82 <0.001 WBC(×109/L) 6.91(4.52~9.82) 7.27(4.40~10.33) Z=-0.59 0.557 NEU(×109/L) 5.00(2.78~7.59) 5.83(3.26~8.23) Z=-1.05 0.292 Hb(g/L) 110.00(88.00~129.00) 112.00(83.00~126.00) Z=-0.37 0.714 PLT(×109/L) 76.00(53.00~107.00) 79.00(54.50~105.00) Z=-0.52 0.606 INR 1.84(1.64~2.19) 1.81(1.62~2.15) Z=-0.01 0.996 PTA(%) 35.10(28.40~41.20) 34.90(28.05~42.20) Z=-0.09 0.930 ALT(U/L) 37.00(24.40~66.00) 41.00(25.00~72.95) Z=-0.51 0.613 AST(U/L) 80.00(52.00~116.00) 76.00(31.35~160.15) Z=-0.74 0.459 PAB(mg/dL) 3.70(2.20~5.70) 3.50(2.40~5.10) Z=-0.24 0.809 Alb(g/L) 26.30(22.30~30.00) 26.50(23.45~30.40) Z=-0.96 0.335 GLO(g/L) 34.13±10.85 31.14±9.01 t=1.50 0.137 TBil(μmol/L) 186.30(117.10~356.40) 256.80(128.40~414.60) Z=-1.31 0.192 Ur(mmol/L) 5.80(3.55~8.40) 8.41(4.80~15.04) Z=-3.48 <0.001 Cr(μmol/L) 63.00(49.00~93.00) 87.00(65.00~118.50) Z=-2.87 0.004 Cys-C(mg/L) 1.21(0.96~1.55) 1.72(1.31~2.26) Z=-4.42 <0.001 K+(mmol/L) 3.62±0.71 4.06±1.04 t=-2.82 0.006 Na+(mmol/L) 136.00(132.00~137.70) 134.00(130.00~137.00) Z=-1.39 0.164 GLU(mmol/L) 5.60(4.70~7.20) 5.80(4.76~8.30) Z=-0.65 0.519 TC(mmol/L) 2.11(1.54~2.86) 2.00(1.52~2.99) Z=-0.03 0.977 TG(mmol/L) 0.81(0.61~1.43) 0.78(0.55~1.28) Z=-0.78 0.435 HDL-C(mmol/L) 0.25(0.13~0.45) 0.15(0.09~0.49) Z=-1.36 0.174 MELD评分(分) 20.00(16.00~23.00) 22.00(17.50~28.00) Z=-2.14 0.032 MELD-Na评分(分) 21.00(18.00~27.00) 28.00(20.50~33.00) Z=-2.71 0.007 MELD 3.0评分(分) 26.00(22.00~30.00) 28.00(24.00~32.00) Z=-1.81 0.070 COSSH-ACLFⅡ评分(分) 7.00(6.00~8.00) 8.00(7.00~9.00) Z=-4.09 <0.001 注:HBV,乙型肝炎病毒;HE,肝性脑病;SBP,自发性细菌性腹膜炎;WBC,白细胞计数;NEU,中性粒细胞计数;Hb,血红蛋白;PLT,血小板计数;INR,国际标准化比值;PTA,凝血酶原活动度;ALT,丙氨酸氨基转移酶;AST,天冬氨酸氨基转移酶;PAB,前白蛋白;Alb,白蛋白;GLO,球蛋白;TBil,总胆红素;Ur,尿素;Cr,肌酐;Cys-C,胱抑素-C;GLU,血糖;TC,总胆固醇;TG,甘油三酯;HDL-C,高密度脂蛋白胆固醇。
表 3 多因素Logistic回归分析
Table 3. Multi-factor Logisitc regression of training set
指标 β值 SE Wald OR(95%CI) P值 糖尿病 1.763 0.664 7.051 5.831(1.587~21.424) 0.008 Cys-C 1.093 0.351 9.725 2.984(1.501~5.933) 0.002 SBP 1.739 0.497 12.254 5.692(2.150~15.071) <0.001 注:Cys-C,胱抑素-C;SBP,自发性细菌性腹膜炎;OR,比值比;CI,置信区间。
-
[1] Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association; Nutrition and Regeneration in End-Stage Liver Disease Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the diagnosis and treatment of acute-on-chronic liver failure(2025 version)[J]. J Prac Hepatol, 2025, 28( 5): 641- 647. DOI: 1 0.3969/j.issn.1672-5069.2025.05.001.中华医学会肝病学分会重型肝病与人工肝学组, 中华医学会肝病学分会终末期肝病营养与再生学组. 慢加急性肝衰竭诊治指南(2025年版)[J]. 实用肝脏病杂志, 2025, 28( 5): 641- 647. DOI: 10.3969/j.issn.1672-5069.2025.05.001. [2] MEZZANO G, JUANOLA A, CARDENAS A, et al. Global burden of disease: Acute-on-chronic liver failure, a systematic review and meta-analysis[J]. Gut, 2022, 71( 1): 148- 155. DOI: 10.1136/gutjnl-2020-322161. [3] LUO JJ, LI JQ, LI P, et al. Acute-on-chronic liver failure: Far to go-a review[J]. Crit Care, 2023, 27( 1): 259. DOI: 10.1186/s13054-023-04540-4. [4] MOORE O, MA WS, READ S, et al. The unwell patient with advanced chronic liver disease: When to use each score?[J]. BMC Med, 2025, 23( 1): 413. DOI: 10.1186/s12916-025-04185-w. [5] GÜLCICEGI DE, GOESER T, KASPER P. Prognostic assessment of liver cirrhosis and its complications: Current concepts and future perspectives[J]. Front Med, 2023, 10: 1268102. DOI: 10.3389/fmed.2023.1268102. [6] LAI RM, CHEN TB, HU YH, et al. Effect of type 2 diabetic mellitus in the prognosis of acute-on-chronic liver failure patients in China[J]. World J Gastroenterol, 2021, 27( 23): 3372- 3385. DOI: 10.3748/wjg.v27.i23.3372. [7] LUO WL, ZENG Y, ZHANG XM, et al. Influence of metabolism-related factors on the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure and establishment of a predictive model[J]. J Clin Hepatol, 2024, 40( 10): 1985- 1991. DOI: 10.12449/JCH241010.罗文苓, 曾玉, 张雪媚, 等. 代谢相关因素对HBV相关慢加急性肝衰竭患者短期预后的影响及预测模型构建[J]. 临床肝胆病杂志, 2024, 40( 10): 1985- 1991. DOI: 10.12449/JCH241010. [8] FU YF, WU KN, YANG SC, et al. Exploring the predictive value of serum lipids for short-term prognosis in patients with acute-on-chronic liver failure[J]. Portal Hypertens Cirrhosis, 2024, 3( 4): 184- 195. DOI: 10.1002/poh2.93. [9] CHEN L, DAI JJ, XIE Q, et al. Metabolic risk factors are associated with the disease severity and prognosis of hepatitis B virus-related acute on chronic liver failure[J]. Gut Liver, 2022, 16( 3): 456- 464. DOI: 10.5009/gnl210449. [10] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. [11] PIANO S, MAHMUD N, CARACENI P, et al. Mechanisms and treatment approaches for ACLF[J]. Liver Int, 2025, 45( 3): e15733. DOI: 10.1111/liv.15733. [12] LAI M, XU MM, WANG X, et al. Prognostic evaluation of liver transplantation for acute-on-chronic liver failure[J]. Organ Transplant, 2025, 16( 3): 482- 488.赖曼, 徐曼曼, 王鑫, 等. 慢加急性肝衰竭肝移植预后评估[J]. 器官移植, 2025, 16( 3): 482- 488. [13] DUSEJA A, DE A, TANEJA S, et al. Impact of metabolic risk factors on the severity and outcome of patients with alcohol-associated acute-on-chronic liver failure[J]. Liver Int, 2021, 41( 1): 150- 157. DOI: 10.1111/liv.14671. [14] LIU C, SHEN J, LI J, et al. DiabetesLiver score: A non-invasive algorithm for advanced liver fibrosis and liver-related outcomes in type 2 diabetes mellitus population[J]. Med, 2025, 6( 8): 100700. DOI: 10.1016/j.medj.2025.100700. [15] KUMAR A, ARORA A, CHOUDHURY A, et al. Impact of diabetes, drug-induced liver injury, and sepsis on outcomes in metabolic dysfunction associated fatty liver disease-related acute-on-chronic liver failure[J]. Am J Gastroenterol, 2025, 120( 4): 816- 826. DOI: 10.14309/ajg.000000-0000002951. [16] YE SY, QIN Y. Research progress on the mechanism of diabetes-induced liver injury[J]. Chin Hepatol, 2023, 28( 6): 737- 739. DOI: 10.14000/j.cnki.issn.1008-1704.2023.06.022.叶圣莹, 秦燕. 糖尿病致肝损伤机制的研究进展[J]. 肝脏, 2023, 28( 6): 737- 739. DOI: 10.14000/j.cnki.issn.1008-1704.2023.06.022. [17] ZHANG J, FAN JG. Expert consensus on the management of diabetes mellitus in patients with liver cirrhosis[J]. J Pract Hepatol, 2022, 25( 5): 761- 775.张晶, 范建高. 肝硬化合并糖尿病患者血糖管理专家共识[J]. 实用肝脏病杂志, 2022, 25( 5): 761- 775. [18] XU Z, ZHANG X, CHEN J, et al. Bacterial infections in acute-on-chronic liver failure: epidemiology, diagnosis, pathogenesis, and management[J]. J Clin Transl Hepatol, 2024, 12( 7): 667- 676. DOI: 10.14218/JCTH.2024.00137. [19] HASSAN A, BHATTI R, HAFEEZ A, et al. Prediction of in-hospital mortality in spontaneous bacterial peritonitis patients with advanced liver disease[J]. Pak J Med Health Sci, 2023, 17( 4): 519- 522. DOI: 10.53350/pjmhs2023174519. [20] LI SM, LIU J, WU J, et al. Immunological mechanisms and effects of bacterial infections in acute-on-chronic liver failure[J]. Cells, 2025, 14( 10): 718. DOI: 10.3390/cells14100718. [21] PHILIPS CA, AUGUSTINE P. Gut barrier and microbiota in cirrhosis[J]. J Clin Exp Hepatol, 2022, 12( 2): 625- 638. DOI: 10.1016/j.jceh.2021.08.027. [22] de OLIVEIRA COBERLLINI JACQUES R, SILVA MASSIGNAN L DA, WINKLER MS, et al. Acute-on-chronic liver failure is independently associated with lower survival in patients with spontaneous bacterial peritonitis[J]. Arq Gastroenterol, 2021, 58( 3): 344- 352. DOI: 10.1590/s0004-2803.202100000-58. [23] MAIWALL R, SINGH SP, ANGELI P, et al. APASL clinical practice guidelines on the management of acute kidney injury in acute-on-chronic liver failure[J]. Hepatol Int, 2024, 18( 3): 833- 869. DOI: 10.1007/s12072-024-10650-0. [24] SAHA R, SHARMA S, MONDAL A, et al. Evaluation of acute kidney injury(AKI) biomarkers FABP1, NGAL, cystatin C and IL-18 in an Indian cohort of hospitalized acute-on-chronic liver failure(ACLF) patients[J]. J Clin Exp Hepatol, 2025, 15( 3): 102491. DOI: 10.1016/j.jceh.2024.102491. [25] IQBAL R, GADDAM M, MOUSTAFA A, et al. The clinical utility of cystatin C in detecting renal dysfunction in cirrhosis: overcoming the limitations of serum creatinine[J]. AAnn Med Surg, 2024. DOI: 10.1097/MS9.0000-000000002822. [26] AUMPAN N, LIMPRUKKASEM T, PORNTHISARN B, et al. Plasma cystatin C level is a prognostic marker of morbidity and mortality in hospitalized decompensated cirrhotic patients[J]. J Med Invest, 2021, 68( 3.4): 302- 308. DOI: 10.2152/jmi.68.302. -



 PDF下载 ( 86519 KB)
PDF下载 ( 86519 KB)

 下载:
下载: