髓系细胞在肝纤维化中的作用及其机制
DOI: 10.12449/JCH260123
-
摘要: 肝纤维化是由多种慢性致病因素引起的以肝脏细胞外基质过度沉积、肝脏结构和功能异常为特征的复杂动态过程。若不及时进行抗纤维化治疗,可进展为肝硬化乃至肝癌。肝纤维化的发病机制复杂,既往研究多集中于肝星状细胞活化。而近年研究发现,髓系细胞因具有多向分化的潜能,亦可参与肝纤维化的发生发展。本文系统综述了髓系细胞在肝纤维化中的作用及调控机制,以期为临床诊断及靶向治疗提供科学参考依据。Abstract: Hepatic fibrosis is a complex dynamic process caused by multiple chronic pathogenic factors, characterized by excessive accumulation of liver extracellular matrix and abnormal liver structure and function. If anti-fibrotic treatment is not performed in time, it can progress to liver cirrhosis and even liver cancer. Hepatic fibrosis has a complex pathogenesis, and previous studies mainly focused on the activation of hepatic stellate cells. Recent studies have shown that myeloid cells have the potential of multi-directional differentiation and can also participate in the development and progression of hepatic fibrosis. This article systematically reviews the role and regulatory mechanism of myeloid cells in hepatic fibrosis, in order to provide a reference for clinical diagnosis and targeted therapy.
-
Key words:
- Hepatic Fibrosis /
- Myeloid Cells /
- Mechanism
-
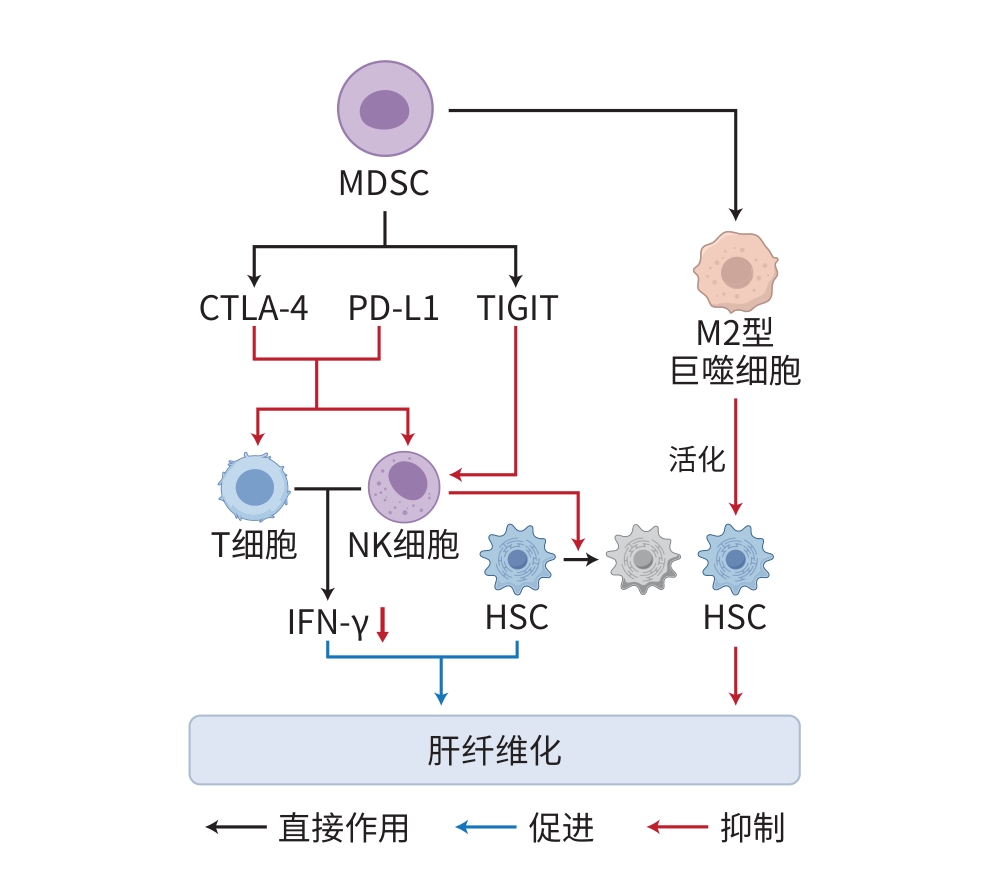
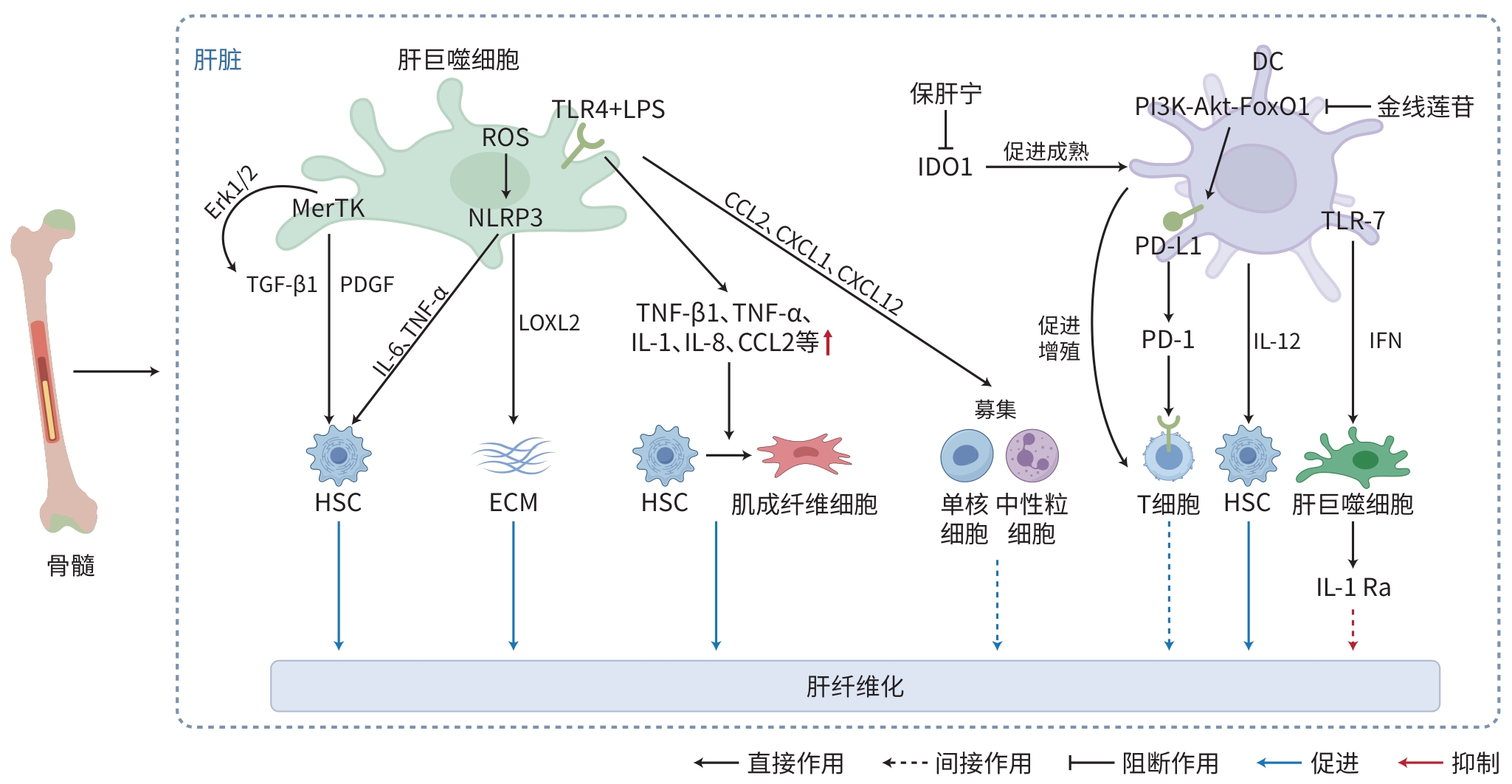
注: MerTK,原癌基因酪氨酸激酶;Erk,外细胞质信号调节激酶;TGF,转化生长因子;PDGF,血小板衍生生长因子;ROS,活性氧;NLRP3,NOD样受体热蛋白结构域相关蛋白3;TNF,肿瘤坏死因子;TLR,Toll样受体;LPS,脂多糖;IL,白细胞介素;LOXL,赖氨酰氧化酶样蛋白;CCL,C-C趋化因子配体;CXCL,C-X-C基序趋化因子配体;HSC,肝星状细胞;ECM,细胞外基质;DC,树突状细胞;IDO1,吲哚胺2,3-双加氧酶1;PI3K,磷脂酰肌醇3激酶;AKT,蛋白激酶B;FoxO1,叉头盒O1;PD-L1,程序性死亡-配体1;PD-1,程序性死亡蛋白受体-1;IFN,干扰素;IL-1Ra,IL-1受体拮抗剂。
图 1 Kupffer细胞及DC在肝纤维化中的作用机制
Figure 1. Mechanism of Kupffer cells and dendritic cells in hepatic fibrosis
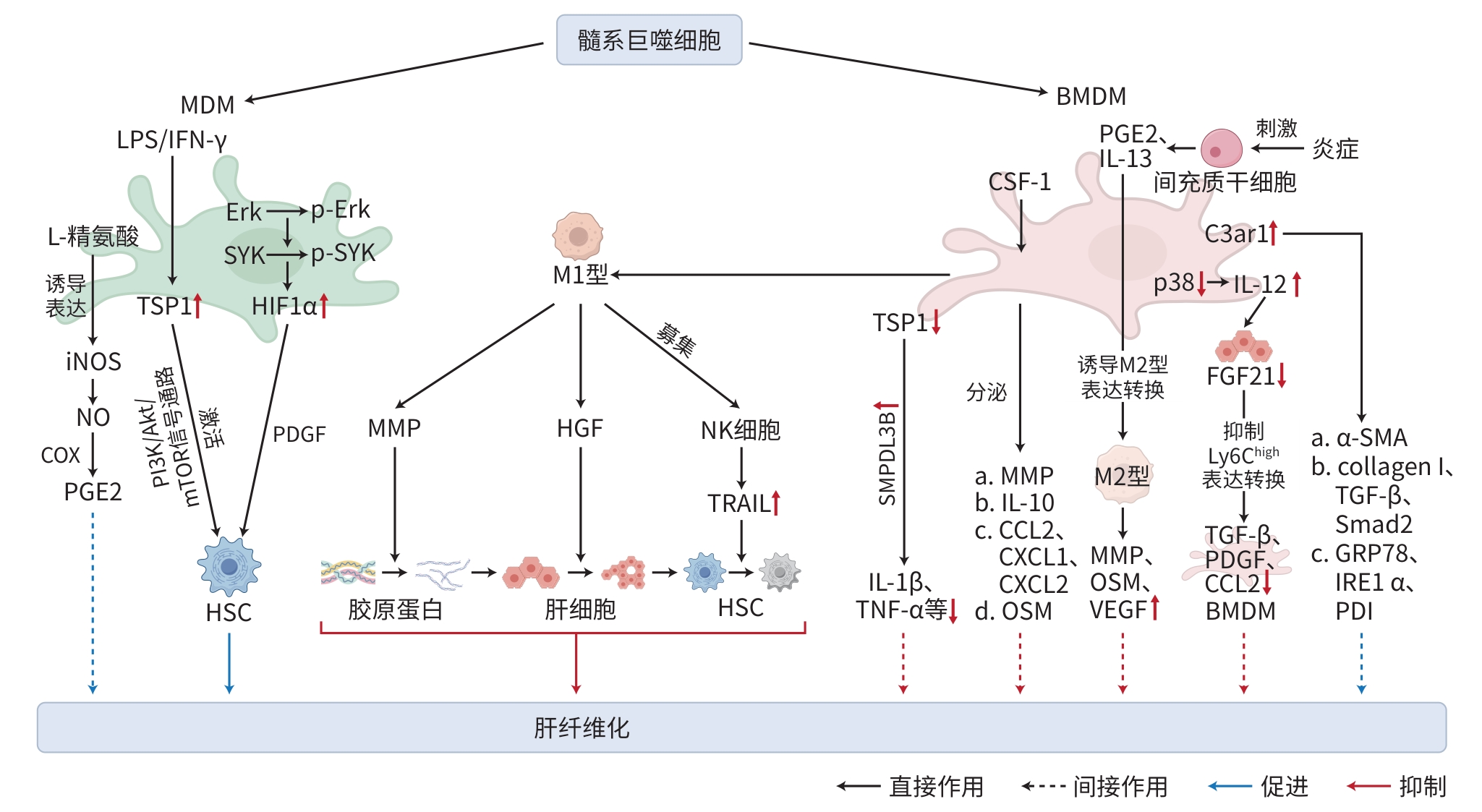
注: MDM,单核细胞来源巨噬细胞;LPS,脂多糖;iNOS,诱导型一氧化氮合酶;NO,一氧化氮;PGE2,前列腺素E2;IFN-γ,干扰素γ;TSP1,血小板反应蛋白1;Erk,外细胞质信号调节激酶;mTOR,雷帕霉素靶蛋白;SYK,脾酪氨酸激酶;HIF1α,缺氧诱导因子1α;PDGF,血小板衍生生长因子;HSC,肝星状细胞;BMDM,骨髓来源巨噬细胞;MMP,基质金属蛋白酶;HGF,肝生长因子;NK细胞,自然杀伤细胞;TRAIL,TNF相关凋亡诱导配体;SMPDL3B;鞘磷脂磷酸二酯酶样3B;IL,白细胞介素;TNF,肿瘤坏死因子;CSF1,集落刺激因子1;CCL,C-C驱化因子配体;CXCL,C-X-C基序驱化因子配体;OSM,抑瘤素M;VEGF,血管内皮生长因子;TGF-β,转化生长因子β;FGF21,成纤维细胞生长因子21;C3ar1,肝脏补体3a受体1;α-SMA,α平滑肌肌动蛋白;collagen Ⅰ,胶原蛋白Ⅰ;GRP78,葡萄糖调节蛋白78;IRE1α,肌醇需求酶1α;PDI,蛋白二硫键异构酶。
图 2 髓系巨噬细胞在肝纤维化中的作用机制
Figure 2. Mechanism of myeloid macrophages in hepatic fibrosis
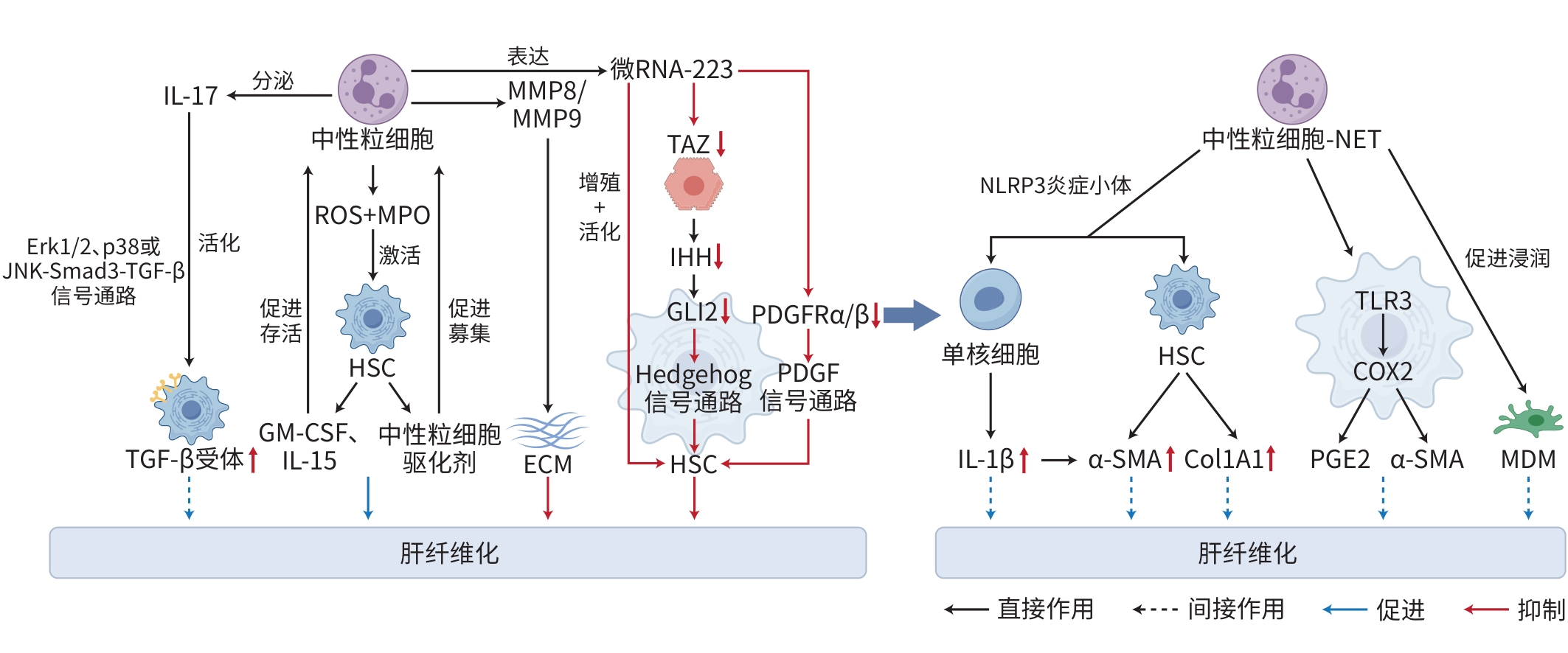
注: JNK,c-Jun氨基末端激酶;TGF-β,转化生长因子-β;IL,白细胞介素;MMP,基质金属蛋白酶;PDGF,血小板衍生生长因子;α-SMA,α平滑肌肌动蛋白;PGE2,前列腺素E2;NLRP3,NOD样受体热蛋白结构域相关蛋白3;SMPDL3B,鞘磷脂磷酸二酯酶样3B;TLR:Toll样受体;ROS,活性氧;MPO,髓过氧化物酶;TAZ,具有PDZ结合序列的转录共激活因子;IHH,印度刺猬蛋白;GLI2,GLI锌指2;NET,中性粒细胞外陷阱;Col1A1,Ⅰ型胶原蛋白α1链;COX2,环氧化酶2;MDM,单核细胞来源巨噬细胞;HSC,肝星状细胞;ECM,细胞外基质;GM-CSF,粒细胞-巨噬细胞集落刺激因子;Erk,外细胞质信号调节激酶。
图 3 中性粒细胞在肝纤维化中的作用机制
Figure 3. Mechanism of neutrophils in hepatic fibrosis
-
[1] Liver Disease Committee, Chinese Association of Integrative Medicine. Guidelines for diagnosis and treatment of liver fibrosis with integrated traditional Chinese and western medicine(2019 edition)[J]. J Chin Hepatol, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007.中国中西医结合学会肝病专业委员会. 肝纤维化中西医结合诊疗指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007. [2] YAMASHITA M, PASSEGUÉ E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration[J]. Cell Stem Cell, 2019, 25( 3): 357- 372. e 7. DOI: 10.1016/j.stem.2019.05.019. [3] BOZHILOV YK, HSU I, BROWN EJ, et al. In vitro human haematopoietic stem cell expansion and differentiation[J]. Cells, 2023, 12( 6): 896. DOI: 10.3390/cells12060896. [4] PIETRAS EM, MIRANTES-BARBEITO C, FONG S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal[J]. Nat Cell Biol, 2016, 18( 6): 607- 618. DOI: 10.1038/ncb3346. [5] MOSSADEGH-KELLER N, SARRAZIN S, KANDALLA PK, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells[J]. Nature, 2013, 497( 7448): 239- 243. DOI: 10.1038/nature12026. [6] ZHANG P, XU LM, GAO JS, et al. 3D collagen matrices modulate the transcriptional trajectory of bone marrow hematopoietic progenitors into macrophage lineage commitment[J]. Bioact Mater, 2022, 10: 255- 268. DOI: 10.1016/j.bioactmat.2021.08.032. [7] WAN LF, PAN WT, YONG YT, et al. Research progress of single-cell transcriptome sequencing technology in liver fibrosis[J]. Curr Biotechnol, 2024, 14( 5): 793- 804. DOI: 10.19586/j.2095-2341.2024.0078.万令飞, 潘文婷, 雍雨婷, 等. 单细胞转录组测序技术在肝纤维化中的研究进展[J]. 生物技术进展, 2024, 14( 5): 793- 804. DOI: 10.19586/j.2095-2341.2024.0078. [8] GUO JB. Effects of constitutive TL1A expression on myeloid cells in liver fibrogenesis and its reversal in mice[D]. Shijiazhuang: Hebei Medical University, 2016. DOI: 10.7666/d.D843844.郭金波. 髓系细胞高表达TL1A在实验性小鼠肝纤维化发生和逆转过程中作用的研究[D]. 石家庄: 河北医科大学, 2016. DOI: 10.7666/d.D843844. [9] CALCAGNO DM, CHU A, GAUL S, et al. NOD-like receptor protein 3 activation causes spontaneous inflammation and fibrosis that mimics human NASH[J]. Hepatology, 2022, 76( 3): 727- 741. DOI: 10.1002/hep.32320. [10] CAI BS, DONGIOVANNI P, COREY KE, et al. Macrophage MerTK promotes liver fibrosis in nonalcoholic steatohepatitis[J]. Cell Metab, 2020, 31( 2): 406- 421. e 7. DOI: 10.1016/j.cmet.2019.11.013. [11] LI WY, CHANG N, LI LY. Heterogeneity and function of kupffer cells in liver injury[J]. Front Immunol, 2022, 13: 940867. DOI: 10.3389/fimmu.2022.940867. [12] SLEVIN E, BAIOCCHI L, WU N, et al. Kupffer cells: Inflammation pathways and cell-cell interactions in alcohol-associated liver disease[J]. Am J Pathol, 2020, 190( 11): 2185- 2193. DOI: 10.1016/j.ajpath.2020.08.014. [13] GUILLOT A, TACKE F. Liver macrophages: Old dogmas and new insights[J]. Hepatol Commun, 2019, 3( 6): 730- 743. DOI: 10.1002/hep4.1356. [14] KOYAMA Y, BRENNER DA. Liver inflammation and fibrosis[J]. J Clin Invest, 2017, 127( 1): 55- 64. DOI: 10.1172/JCI88881. [15] KALLIS YN, SCOTTON CJ, MACKINNON AC, et al. Proteinase activated receptor 1 mediated fibrosis in a mouse model of liver injury: A role for bone marrow derived macrophages[J]. PLoS One, 2014, 9( 1): e86241. DOI: 10.1371/journal.pone.0086241. [16] LI J, LIU WQ, ZHANG J, et al. The role of mitochondrial quality control in liver diseases: Dawn of a therapeutic era[J]. Int J Biol Sci, 2025, 21( 4): 1767- 1783. DOI: 10.7150/ijbs.107777. [17] ZHAO SX, LI WC, FU N, et al. CD14+ monocytes and CD163+ macrophages correlate with the severity of liver fibrosis in patients with chronic hepatitis C[J]. Exp Ther Med, 2020, 20( 6): 228. DOI: 10.3892/etm.2020.9358. [18] XIANG M, LIU TT, TIAN C, et al. Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway[J]. Pharmacol Res, 2022, 177: 106092. DOI: 10.1016/j.phrs.2022.106092. [19] MO C, XIE SW, GAO L, et al. Baoganning formula alleviates liver fibrosis in mice by inhibiting hepatic IDO1 expression and promoting phenotypic maturation of dendritic cells[J]. J South Med Univ, 2021, 41( 7): 1002- 1011. DOI: 10.12122/j.issn.1673-4254.2021.07.06.莫婵, 谢淑雯, 高磊, 等. 复方保肝宁减轻小鼠肝纤维化的机制: 抑制肝脏组织中的IDO1进而促进树突状细胞表型成熟[J]. 南方医科大学学报, 2021, 41( 7): 1002- 1011. DOI: 10.12122/j.issn.1673-4254.2021.07.06. [20] LI T, LIU HB, HU WY, et al. Role of inflammation in hepatic fibrosis[J]. J Clin Hepatol, 2022, 38( 10): 2368- 2372. DOI: 10.3969/j.issn.1001-5256.2022.10.032.李婷, 刘华宝, 胡文艳, 等. 炎症在肝纤维化中的作用[J]. 临床肝胆病杂志, 2022, 38( 10): 2368- 2372. DOI: 10.3969/j.issn.1001-5256.2022.10.032. [21] HORN P, TACKE F. Metabolic reprogramming in liver fibrosis[J]. Cell Metab, 2024, 36( 7): 1439- 1455. DOI: 10.1016/j.cmet.2024.05.003. [22] MOORING M, YEUNG GA, LUUKKONEN P, et al. Hepatocyte CYR61 polarizes profibrotic macrophages to orchestrate NASH fibrosis[J]. Sci Transl Med, 2023, 15( 715): eade3157. DOI: 10.1126/scitranslmed.ade3157. [23] HOU C, WANG D, ZHAO MX, et al. MANF brakes TLR4 signaling by competitively binding S100A8 with S100A9 to regulate macrophage phenotypes in hepatic fibrosis[J]. Acta Pharm Sin B, 2023, 13( 10): 4234- 4252. DOI: 10.1016/j.apsb.2023.07.027. [24] RAN JQ, YIN SX, ISSA R, et al. Key role of macrophages in the progression of hepatic fibrosis[J]. Hepatol Commun, 2025, 9( 1): e0602. DOI: 10.1097/hc9.0000000000000602. [25] CHENG S, ZOU YH, ZHANG M, et al. Single-cell RNA sequencing reveals the heterogeneity and intercellular communication of hepatic stellate cells and macrophages during liver fibrosis[J]. MedComm, 2023, 4( 5): e378. DOI: 10.1002/mco2.378. [26] CHEN XJ, WANG ZY, HAN S, et al. Targeting SYK of monocyte-derived macrophages regulates liver fibrosis via crosstalking with Erk/Hif1α and remodeling liver inflammatory environment[J]. Cell Death Dis, 2021, 12( 12): 1123. DOI: 10.1038/s41419-021-04403-2. [27] BRENIG R, POP OT, TRIANTAFYLLOU E, et al. Expression of AXL receptor tyrosine kinase relates to monocyte dysfunction and severity of cirrhosis[J]. Life Sci Alliance, 2020, 3( 1): e201900465. DOI: 10.26508/lsa.201900465. [28] HAMMERICH L, TACKE F. Hepatic inflammatory responses in liver fibrosis[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 10): 633- 646. DOI: 10.1038/s41575-023-00807-x. [29] MA PF, GAO CC, YI J, et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice[J]. J Hepatol, 2017, 67( 4): 770- 779. DOI: 10.1016/j.jhep.2017.05.022. [30] GWAG T, REDDY MOOLI RG, LI D, et al. Macrophage-derived thrombospondin 1 promotes obesity-associated non-alcoholic fatty liver disease[J]. JHEP Rep, 2021, 3( 1): 100193. DOI: 10.1016/j.jhepr.2020.100193. [31] WATANABE Y, TSUCHIYA A, SEINO S, et al. Mesenchymal stem cells and induced bone marrow-derived macrophages synergistically improve liver fibrosis in mice[J]. Stem Cells Transl Med, 2019, 8( 3): 271- 284. DOI: 10.1002/sctm.18-0105. [32] CRESPO M, NIKOLIC I, MORA A, et al. Myeloid p38 activation maintains macrophage-liver crosstalk and BAT thermogenesis through IL-12-FGF21 axis[J]. Hepatology, 2023, 77( 3): 874- 887. DOI: 10.1002/hep.32581. [33] LI ST, ZHOU B, XUE M, et al. Macrophage-specific FGF12 promotes liver fibrosis progression in mice[J]. Hepatology, 2023, 77( 3): 816- 833. DOI: 10.1002/hep.32640. [34] HAN JQ, ZHANG X, LAU JK, et al. Bone marrow-derived macrophage contributes to fibrosing steatohepatitis through activating hepatic stellate cells[J]. J Pathol, 2019, 248( 4): 488- 500. DOI: 10.1002/path.5275. [35] TANG JJ, YAN ZJ, FENG QY, et al. The roles of neutrophils in the pathogenesis of liver diseases[J]. Front Immunol, 2021, 12: 625472. DOI: 10.3389/fimmu.2021.625472. [36] SAIJOU E, ENOMOTO Y, MATSUDA M, et al. Neutrophils alleviate fibrosis in the CCl(4)-induced mouse chronic liver injury model[J]. Hepatol Commun, 2018, 2( 6): 703- 717. DOI: 10.1002/hep4.1178. [37] WANG XL, SEO W, PARK SH, et al. microRNA-223 restricts liver fibrosis by inhibiting the TAZ-IHH-GLI2 and PDGF signaling pathways via the crosstalk of multiple liver cell types[J]. Int J Biol Sci, 2021, 17( 4): 1153- 1167. DOI: 10.7150/ijbs.58365. [38] QI JS, PING DB, SUN X, et al. The role of neutrophils in liver fibrosis[J]. Chin Hepatol, 2023, 28( 9): 1127- 1130. DOI: 10.14000/j.cnki.issn.1008-1704.2023.09.029.齐婧姝, 平大冰, 孙鑫, 等. 中性粒细胞在肝纤维化中的作用[J]. 肝脏, 2023, 28( 9): 1127- 1130. DOI: 10.14000/j.cnki.issn.1008-1704.2023.09.029. [39] BABUTA M, MOREL C, DE CARVALHO RIBEIRO M, et al. Neutrophil extracellular traps activate hepatic stellate cells and monocytes via NLRP3 sensing in alcohol-induced acceleration of MASH fibrosis[J]. Gut, 2024, 73( 11): 1854- 1869. DOI: 10.1136/gutjnl-2023-331447. [40] XIA YJ, WANG Y, XIONG Q, et al. Neutrophil extracellular traps promote MASH fibrosis by metabolic reprogramming of HSC[J]. Hepatology, 2025, 81( 3): 947- 961. DOI: 10.1097/HEP.0000000000000762. [41] van der WINDT DJ, SUD V, ZHANG HJ, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis[J]. Hepatology, 2018, 68( 4): 1347- 1360. DOI: 10.1002/hep.29914. [42] ZHOU ZJ, LAI PH, ZHANG SL, et al. The relationship between hepatic myeloid-derived suppressor cells and clinicopathological parameters in patients with chronic liver disease[J]. Biomed Res Int, 2021, 2021: 6612477. DOI: 10.1155/2021/6612477. [43] LI TY, YANG Y, ZHOU G, et al. Immune suppression in chronic hepatitis B infection associated liver disease: A review[J]. World J Gastroenterol, 2019, 25( 27): 3527- 3537. DOI: 10.3748/wjg.v25.i27.3527. [44] SENDO S, SAEGUSA J, MORINOBU A. Myeloid-derived suppressor cells in non-neoplastic inflamed organs[J]. Inflamm Regen, 2018, 38: 19. DOI: 10.1186/s41232-018-0076-7. [45] JI B. Study on the mechanism of myeloid suppressor cells in the occurrence of liver fibrosis in mice[D]. Changchun: Jilin University, 2013.纪柏. 髓样抑制细胞在小鼠肝纤维化发生中的影响机制研究[D]. 长春: 吉林大学, 2013. [46] GUO FY, WANG DW. Research advances on pathogenesis of liver fibrosis and related therapeutic drugs[J]. Prog Pharm Sci, 2024, 48( 11): 838- 848. DOI: 10.20053/j.issn1001-5094.2024.11.004.郭飞宇, 王多伟. 肝纤维化发生机制及相关治疗药物研究进展[J]. 药学进展, 2024, 48( 11): 838- 848. DOI: 10.20053/j.issn1001-5094.2024.11.004. [47] SHEN B, LU LG. Mechanism and therapeutic strategy of macrophage myeloid-epithelial-reproductive tyrosine kinase promoting NASH fibrosis[J]. Chin Hepatol, 2020, 25( 10): 1021- 1023. DOI: 10.14000/j.cnki.issn.1008-1704.2020.10.002.沈波, 陆伦根. 巨噬细胞髓细胞-上皮-生殖酪氨酸激酶促进NASH纤维化的机制及治疗策略[J]. 肝脏, 2020, 25( 10): 1021- 1023. DOI: 10.14000/j.cnki.issn.1008-1704.2020.10.002. [48] XU J, JIN WL, LI X. A new perspective in the treatment of liver fibrosis: Targeting macrophage metabolism[J]. J Clin Hepatol, 2023, 39( 4): 922- 928. DOI: 10.3969/j.issn.1001-5256.2023.04.027.许钧, 金卫林, 李汛. 肝纤维化治疗的新视角: 靶向巨噬细胞代谢[J]. 临床肝胆病杂志, 2023, 39( 4): 922- 928. DOI: 10.3969/j.issn.1001-5256.2023.04.027. -



 PDF下载 ( 1227 KB)
PDF下载 ( 1227 KB)

 下载:
下载: