| [1] |
|
| [2] |
PARK JW, CHEN MS, COLOMBO M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study[J]. Liver Int, 2015, 35( 9): 2155- 2166. DOI: 10.1111/liv.12818. |
| [3] |
KWON MJ, CHANG S, KIM JH, et al. Factors associated with the survival outcomes of patients with untreated hepatocellular carcinoma: An analysis of nationwide data[J]. Front Oncol, 2023, 13: 1142661. DOI: 10.3389/fonc.2023.1142661. |
| [4] |
ZHU LZ, XU XL, ABUDUSALAMU AN, et al. Research advances in immunotherapy for hepatocellular carcinoma[J]. J Clin Hepatol, 2023, 39( 5): 1197- 1203. DOI: 10.3969/j.issn.1001-5256.2023.05.031. |
| [5] |
RULI TM Jr, POLLACK ED, LODH A, et al. Immune checkpoint inhibitors in hepatocellular carcinoma and their hepatic-related side effects: A review[J]. Cancers, 2024, 16( 11): 2042. DOI: 10.3390/cancers16112042. |
| [6] |
YU YX, WANG S, LIU ZN, et al. Traditional Chinese medicine in the era of immune checkpoint inhibitor: Theory, development, and future directions[J]. Chin Med, 2023, 18( 1): 59. DOI: 10.1186/s13020-023-00751-7. |
| [7] |
YOUNIS A, GRIBBEN J. Immune checkpoint inhibitors: Fundamental mechanisms, current status and future directions[J]. Immuno, 2024, 4( 3): 186- 210. DOI: 10.3390/immuno4030013. |
| [8] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma(CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389( 10088): 2492- 2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [9] |
FINN RS, QIN SK, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382( 20): 1894- 1905. DOI: 10.1056/NEJMoa1915745. |
| [10] |
SPRANGER S, DAI D, HORTON B, et al. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy[J]. Cancer Cell, 2017, 31( 5): 711- 723. e 4. DOI: 10.1016/j.ccell.2017.04.003. |
| [11] |
POSTOW MA, SIDLOW R, HELLMANN MD. Immune-related adverse events associated with immune checkpoint blockade[J]. N Engl J Med, 2018, 378( 2): 158- 168. DOI: 10.1056/NEJMra1703481. |
| [12] |
MENG XF, WANG XB. Clinical thinking and application of spleen-strengthening liver-protecting and nude eliminating methods for liver cancer used by WANG Xian-bo[J]. Beijing J Tradit Chin Med, 2019, 38( 7): 654- 657. DOI: 10.16025/j.1674-1307.2019.07.007. |
| [13] |
LIU FX, HAN Y, SUN T, et al. Clinical efficacy of a self-formulated Jiedu Shugan Huoxue formula combined with PD-1 inhibitors in treating primary liver cancer after microwave ablation[J]. Acta Chin Med Pharmacol, 2024, 52( 12): 93- 98. DOI: 10.19664/j.cnki.1002-2392.240256. |
| [14] |
ZHANG LJ. Efficacy and safety of hepatoma Ⅰ formula plus minus plus and minus plus PD-1 inhibitor in the treatment of advanced primary hepatoma[D]. Lanzhou: Gansu University of Chinese Medicine, 2023.
张丽娟. 肝癌Ⅰ号方加减联合PD-1抑制剂治疗中晚期原发性肝癌的疗效及安全性[D]. 兰州: 甘肃中医药大学, 2023.
|
| [15] |
ZHOU K, YUAN W, WU J, et al. Clinical efficacy of Shugan Jianpi Jiedu decoction combined with tirelizumab and bevacizumab on patients with advanced liver cancer[J]. Chin Tradit Pat Med, 2024, 46( 12): 4221- 4224. DOI: 10.3969/j.issn.1001-1528.2024.12.052. |
| [16] |
ZHAI FY, XU YQ. Advances in the study of traditional Chinese medicine to prevention and treatment immune-related adverse reactions of PD-1/PD-L1inhibitors[J]. Clin J Tradit Chin Med, 2022, 34( 9): 1773- 1777. DOI: 10.16448/j.cjtcm.2022.0945. |
| [17] |
YU D, HE SL, SHEN J, et al. Clinical observation of Ruyi Jinhuang powder combined with ICIs in the treatment of advanced liver cancer complicated with dampness and heat syndrome of liver and gallbladder[J]. China Pharm, 2023, 34( 12): 1488- 1492. DOI: 10.6039/j.issn.1001-0408.2023.12.15. |
| [18] |
WANG Y. Clinical observation on the therapeutic effect of SiWuXiaoFeng decoction on immune checkpoint inhibitor-associated skin toxicity[D]. Changchun: Changchun University of Chinese Medicine, 2024.
王燕. 四物消风饮治疗免疫检查点抑制剂相关皮肤毒性的临床疗效观察[D]. 长春: 长春中医药大学, 2024.
|
| [19] |
CHEN HZ, LIANG WL, GU Z, et al. Etiology and pathogeny analysis and treatment exploration in traditional Chinese medicine of immune-related adverse reactions of PD-1/PD-L1 inhibitors[J]. World Chin Med, 2021, 16( 9): 1386- 1390, 1399. DOI: 10.3969/j.issn.1673-7202.2021.09.007. |
| [20] |
QIU WL, SUN QQ, QIU B. Action mechanism progress of Chinese medicinal and its active ingredients in treatment of hepatic carcinoma[J]. Inf Tradit Chin Med, 2022, 39( 5): 79- 84. DOI: 10.19656/j.cnki.1002-2406.20220515. |
| [21] |
CHENG QL, LIU L, BAI CC, et al. Mechanism of action of five classic prescriptions in treatment of hepatocellular carcinoma based on network pharmacology[J]. J Clin Hepatol, 2021, 37( 8): 1848- 1855. DOI: 10.3969/j.issn.1001-5256.2021.08.020. |
| [22] |
QIAO XP, LUO C, DING XS, et al. Research progress on active components and anticancer molecular mechanism of Huaier[J]. Chem Life, 2023, 43( 1): 124- 129. DOI: 10.13488/j.smhx.20220772. |
| [23] |
LIU HY, LI X, ZHANG CW, et al. GJB2 promotes HCC progression by activating glycolysis through cytoplasmic translocation and generating a suppressive tumor microenvironment based on single cell RNA sequencing[J]. Adv Sci, 2024, 11( 39): 2402115. DOI: 10.1002/advs.202402115. |
| [24] |
GUO HL, LI J, ZHANG Q, et al. Mechanism of rhizoma curcumae-rhizoma sparganii against hepatocellular carcinoma based on PD-1 mediated NF-κB pathway[J]. J Mod Oncol, 2024, 32( 7): 1179- 1185. DOI: 10.3969/j.issn.1672-4992.2024.07.001. |
| [25] |
GUO L, LI HB, FAN TL, et al. Synergistic efficacy of curcumin and anti-programmed cell death-1 in hepatocellular carcinoma[J]. Life Sci, 2021, 279: 119359. DOI: 10.1016/j.lfs.2021.119359. |
| [26] |
ZHANG M, WANG L, LIU W, et al. Targeting inhibition of accumulation and function of myeloid-derived suppressor cells by artemisinin via PI3K/AKT, mTOR, and mapk pathways enhances anti-PD-L1 immunotherapy in melanoma and liver tumors[J]. J Immunol Res, 2022, 2022: 2253436. DOI: 10.1155/2022/2253436. |
| [27] |
TIAN XC, LIU F, WANG ZJ, et al. Modified Biejia Jianwan decoction restrains PD-L1-mediated immune evasion through the HIF-1α/STAT3/NF-κB signaling pathway[J]. J Ethnopharmacol, 2024, 322: 117577. DOI: 10.1016/j.jep.2023.117577. |
| [28] |
HE L, LIU XP, CHEN GS, et al. Banxia Xiexin decoction inhibits macrophage polarization to M2Phenotype to sensitize hepatocellular carcinoma to PD-1 monoclonal antibody[J]. Pharmacol Clin Chin Mater Med, 2025, 41( 4): 21- 28. DOI: 10.13412/j.cnki.zyyl.20241115.003. |
| [29] |
DING H, WANG J, WANG ZG, et al. Clinical effectiveness of cantharidin-containing Chinese medicine preparations on immune function in patients with hepatocellular cancer: A meta-analysis[J]. J Oncol Chin Med, 2020, 2( 1): 85- 91. DOI: 10.19811/j.cnki.ISSN2096-6628.2020.01.020. |
| [30] |
WU YN, LI X, ZHANG D, et al. Influence of BeilongRuangan decoction on perliferation of peripheral blood dendritic cells from HBV related hepatocellular carcinoma patients[J]. Liaoning J Tradit Chin Med, 2019, 46( 3): 646- 649, 673. DOI: 10.13192/j.issn.1000-1719.2019.03.060. |
| [31] |
LI HW. Efficacy and mechanism of Huaier combined with immune checkpoint inhibitors against hepatocellular carcinoma[J]. Chengdu: Chengdu University of Traditional Chinese Medicine, 2023.
李华伟. 槐耳联合免疫检查点抑制剂治疗肝癌的疗效及机制研究[D]. 成都: 成都中医药大学, 2023.
|
| [32] |
CAO C, LIU ZY, ZHANG X, et al. A clinical study on the effect of Wenyang fuzheng formula combined with PD-1 immunotherapy on the microenvironment of Yang deficiency type of liver cancer[J]. Labeled Immunoass Clin Med, 2024, 31( 6): 1042- 1048. DOI: 10.11748/bjmy.issn.1006-1703.2024.06.012. |
| [33] |
BAGLIERI J, BRENNER DA, KISSELEVA T. The role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma[J]. Int J Mol Sci, 2019, 20( 7): 1723. DOI: 10.3390/ijms20071723. |
| [34] |
AMERATUNGA M, CHÉNARD-POIRIER M, MORENO CANDILEJO I, et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors[J]. Eur J Cancer, 2018, 89: 56- 63. DOI: 10.1016/j.ejca.2017.11.012. |
| [35] |
LIU MK, JI MM, CHENG L, et al. Research progress in anti-tumor effect and mechanism of baicalin[J]. J Shanghai Jiao Tong Univ Med Sci, 2021, 41( 2): 246- 250. DOI: 10.3969/j.issn.1674-8115.2021.02.019. |
| [36] |
TIAN LJ, SANG YJ, SUN YJ, et al. The predictive value of systemic immune-inflammation index for immune checkpoint inhibitor treatment-related adverse reactions in patients with primary liver cancer[J]. J Shandong Univ Health Sci, 2024, 62( 6): 48- 53, 75. DOI: 10.6040/j.issn.1671-7554.0.2024.0267. |
| [37] |
YANG QQ, WANG B, ZHENG QH, et al. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy[J]. Adv Sci, 2023, 10( 15): 2207366. DOI: 10.1002/advs.202207366. |
| [38] |
LEE SH, CHO SY, YOON Y, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice[J]. Nat Microbiol, 2021, 6( 3): 277- 288. DOI: 10.1038/s41564-020-00831-6. |
| [39] |
ZHANG ZQ, SHI XL, JI JM, et al. Dihydroartemisinin increased the abundance of Akkermansia muciniphila by YAP1 depression that sensitizes hepatocellular carcinoma to anti-PD-1 immunotherapy[J]. Front Med, 2023, 17( 4): 729- 746. DOI: 10.1007/s11684-022-0978-2. |



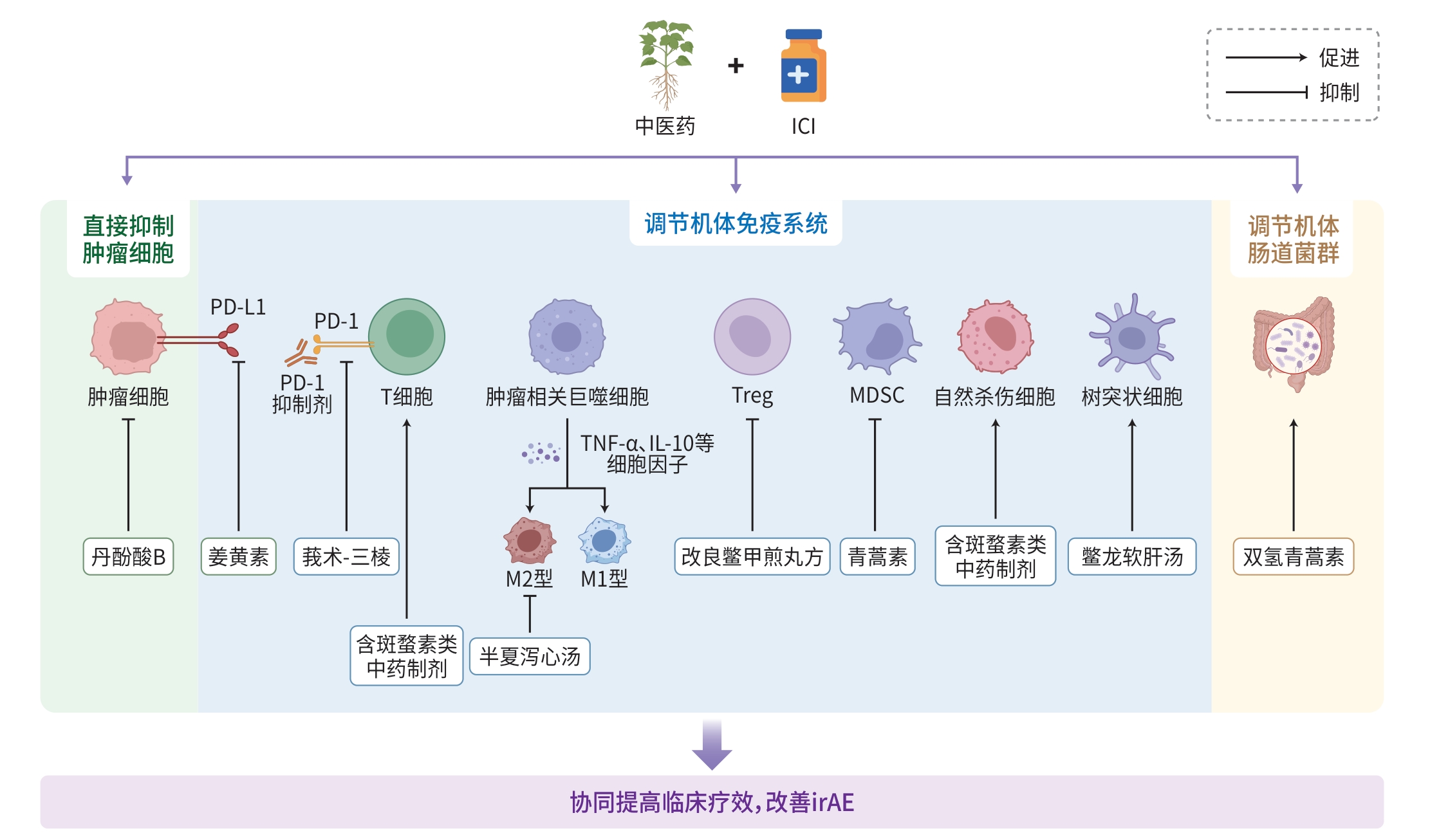




 DownLoad:
DownLoad: