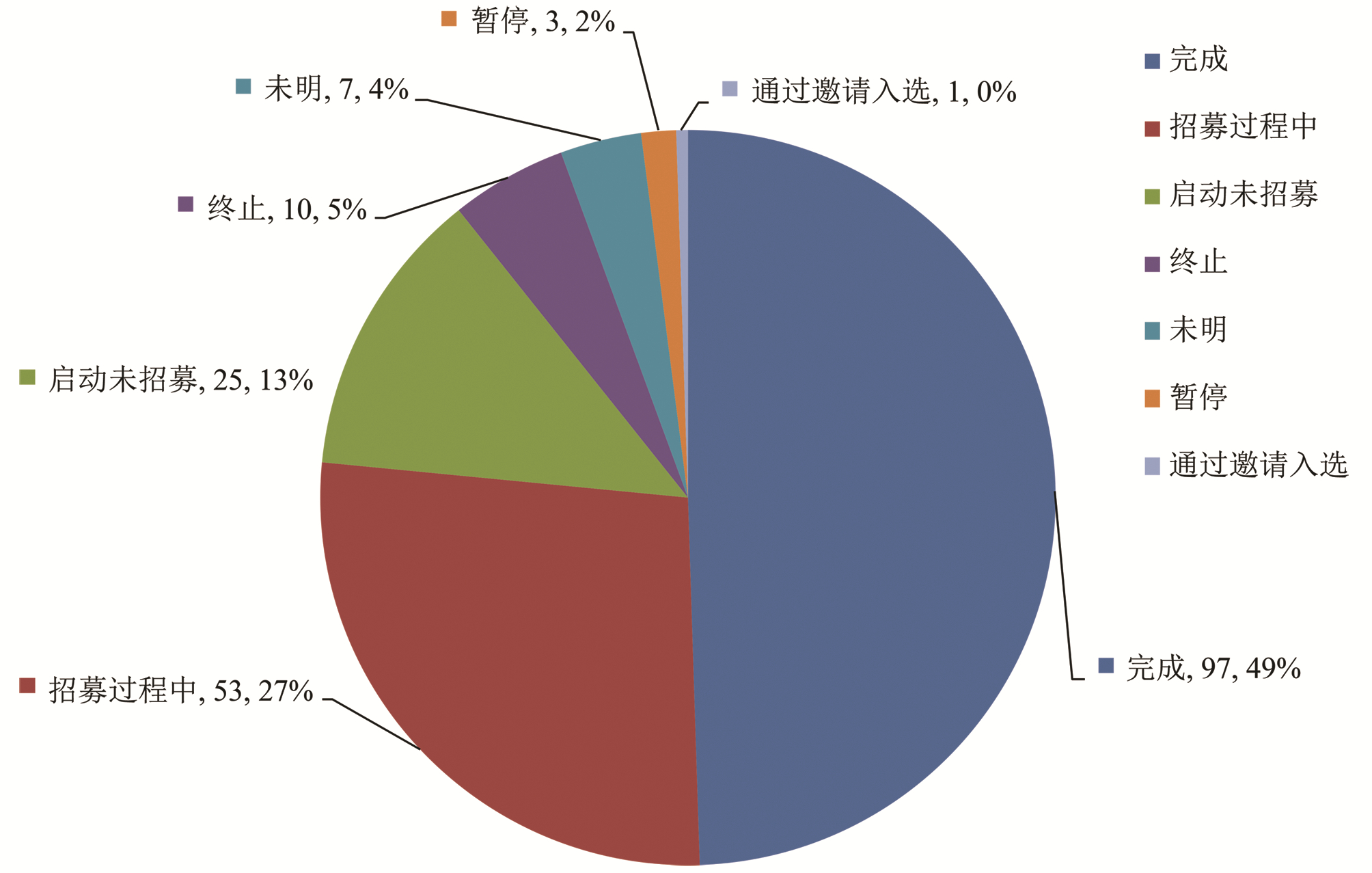
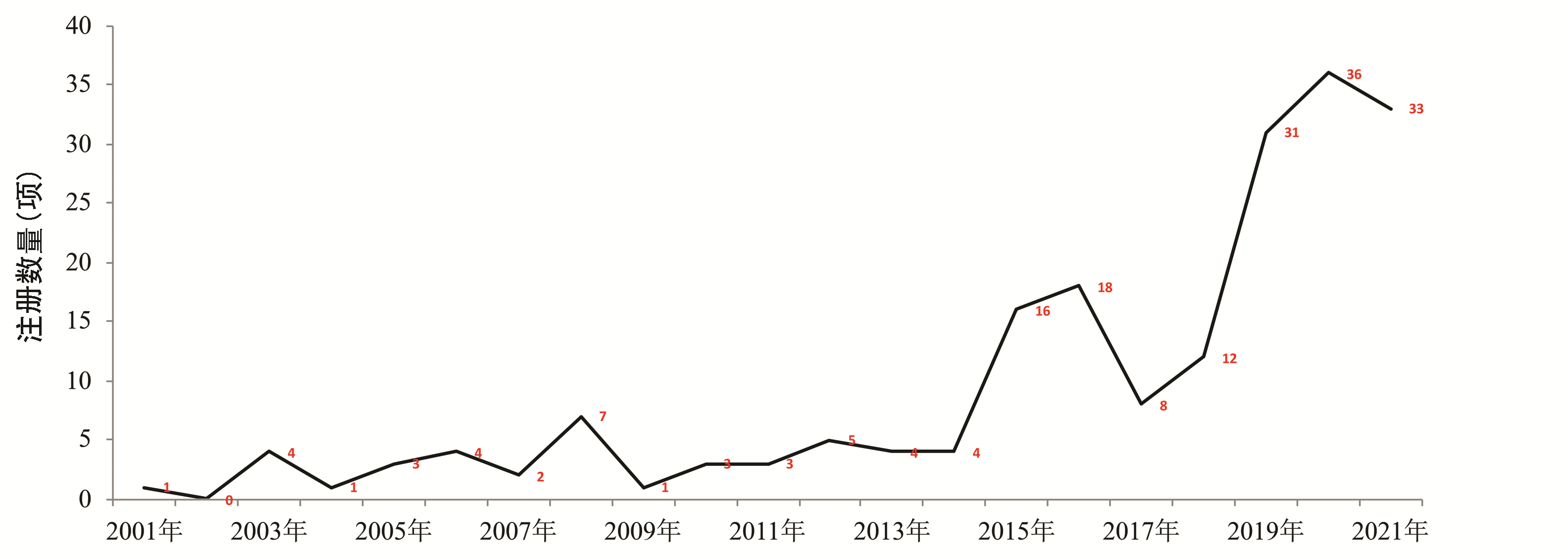
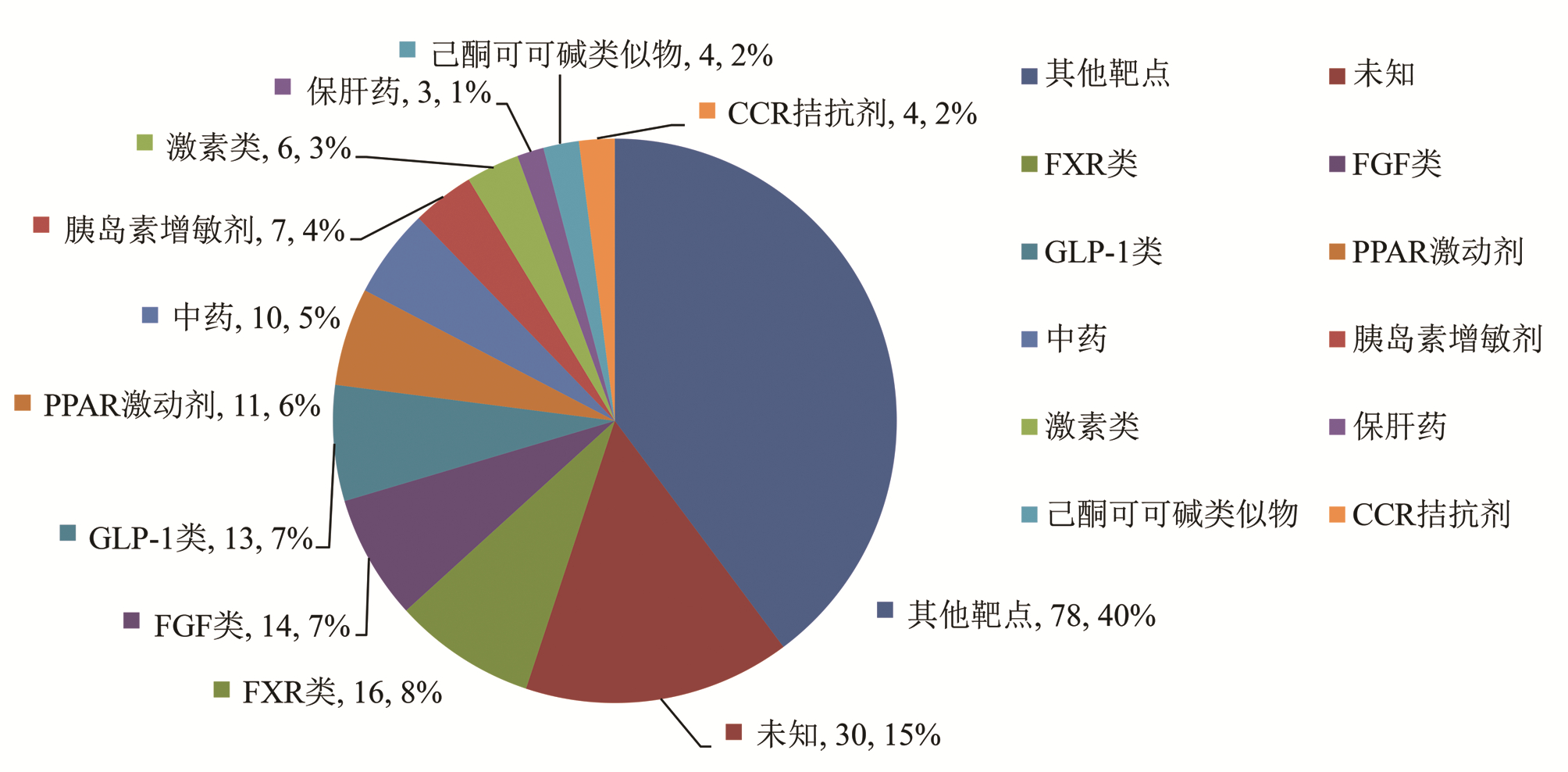
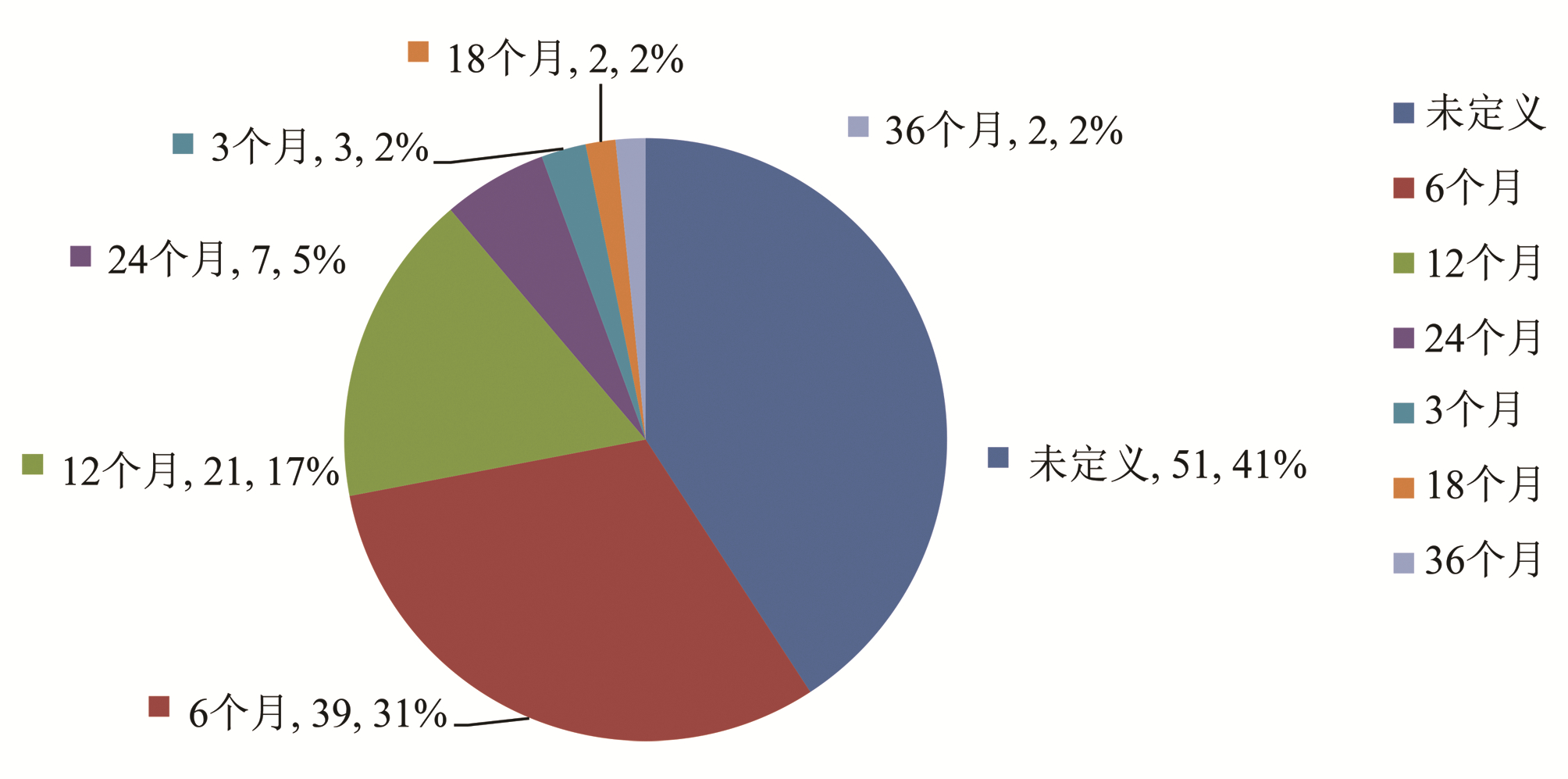
| [1] |
National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34(5): 947-957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. |
| [2] |
YOUNOSSI ZM, KOENIG AB, ABDELATIF D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes[J]. Hepatology, 2016, 64(1): 73-84. DOI: 10.1002/hep.28431. |
| [3] |
SANYAL AJ, FRIEDMAN SL, MCCULLOUGH AJ, et al. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop[J]. Hepatology, 2015, 61(4): 1392-1405. DOI: 10.1002/hep.27678. |
| [4] |
LAZARIDIS N, TSOCHATZIS E. Current and future treatment options in non-alcoholic steatohepatitis (NASH)[J]. Expert Rev Gastroenterol Hepatol, 2017, 11(4): 357-369. DOI: 10.1080/17474124.2017.1293523. |
| [5] |
ANANIA FA, DIMICK-SANTOS L, MEHTA R, et al. Nonalcoholic steatohepatitis: Current thinking from the division of hepatology and nutrition at the food and drug administration[J]. Hepatology, 2021, 73(5): 2023-2027. DOI: 10.1002/hep.31687. |
| [6] |
RINELLA ME, TACKE F, SANYAL AJ, et al. Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD[J]. J Hepatol, 2019, 71(4): 823-833. DOI: 10.1016/j.jhep.2019.04.019. |
| [7] |
|
| [8] |
KLEINER DE, BRUNT EM, van NATTA M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease[J]. Hepatology, 2005, 41(6): 1313-1321. DOI: 10.1002/hep.20701. |
| [9] |
KONERMAN MA, JONES JC, HARRISON SA. Pharmacotherapy for NASH: Current and emerging[J]. J Hepatol, 2018, 68(2): 362-375. DOI: 10.1016/j.jhep.2017.10.015. |
| [10] |
REIMER KC, WREE A, RODERBURG C, et al. New drugs for NAFLD: Lessons from basic models to the clinic[J]. Hepatol Int, 2020, 14(1): 8-23. DOI: 10.1007/s12072-019-10001-4. |
| [11] |
TONG XF, SUN YM, YOU H. Evaluation of clinical endpoints in new drug research and development for nonalcoholic steatohepatitis[J]. J Clin Hepatol, 2021, 37(6): 1249-1253. DOI: 10.3969/j.issn.1001-5256.2021.06.003. |
| [12] |
DAVISON BA, HARRISON SA, COTTER G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials[J]. J Hepatol, 2020, 73(6): 1322-1332. DOI: 10.1016/j.jhep.2020.06.025. |
| [13] |
CAUSSY C, REEDER SB, SIRLIN CB, et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials[J]. Hepatology, 2018, 68(2): 763-772. DOI: 10.1002/hep.29797. |
| [14] |
JAYAKUMAR S, MIDDLETON MS, LAWITZ EJ, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: Analysis of data from a phase Ⅱ trial of selonsertib[J]. J Hepatol, 2019, 70(1): 133-141. DOI: 10.1016/j.jhep.2018.09.024. |
| [15] |
HOOKER JC, HAMILTON G, PARK CC, et al. Inter-reader agreement of magnetic resonance imaging proton density fat fraction and its longitudinal change in a clinical trial of adults with nonalcoholic steatohepatitis[J]. Abdom Radiol (NY), 2019, 44(2): 482-492. DOI: 10.1007/s00261-018-1745-3. |
| [16] |
SASSO M, AUDIÈRE S, KEMGANG A, et al. Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the FibroScan: A Pilot study assessing diagnostic accuracy[J]. Ultrasound Med Biol, 2016, 42(1): 92-103. DOI: 10.1016/j.ultrasmedbio.2015.08.008. |
| [17] |
VILAR-GOMEZ E, MARTINEZ-PEREZ Y, CALZADILLA-BERTOT L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis[J]. Gastroenterology, 2015, 149(2): 367-378. e5; quiz e14-e15. DOI: 10.1053/j.gastro.2015.04.005. |
| [18] |
ROMERO-GÓMEZ M, ZELBER-SAGI S, TRENELL M. Treatment of NAFLD with diet, physical activity and exercise[J]. J Hepatol, 2017, 67(4): 829-846. DOI: 10.1016/j.jhep.2017.05.016. |
| [19] |
SANYAL AJ, BRUNT EM, KLEINER DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis[J]. Hepatology, 2011, 54(1): 344-353. DOI: 10.1002/hep.24376. |
| [20] |
RATZIU V. A critical review of endpoints for non-cirrhotic NASH therapeutic trials[J]. J Hepatol, 2018, 68(2): 353-361. DOI: 10.1016/j.jhep.2017.12.001. |
| [21] |
LICHTINGHAGEN R, PIETSCH D, BANTEL H, et al. The enhanced liver fibrosis (ELF) score: Normal values, influence factors and proposed cut-off values[J]. J Hepatol, 2013, 59(2): 236-242. DOI: 10.1016/j.jhep.2013.03.016. |








 DownLoad:
DownLoad: