丙型肝炎病毒感染的实验室检测方法及策略
DOI: 10.12449/JCH240405
Advances in laboratory testing methods and strategies for hepatitis C virus infection
-
摘要: 丙型肝炎病毒(HCV)感染的实验室检测为HCV感染者的检测发现和诊断提供关键依据。近年来,HCV感染检测技术不断发展,检测试剂性能显著提高,新的检测服务策略不断出现并逐步推广应用。本文归纳了国内外HCV感染的实验室检测方法及检测策略,总结了HCV感染检测的方法,分析了新方法和新策略对我国HCV感染防控带来的影响。及时准确的实验室检测方法和有效可行的检测策略有助于HCV感染的早发现、早诊断、早治疗,最终实现2030年消除病毒性肝炎主要公共卫生危害的战略目标。Abstract: Laboratory testing for hepatitis C virus (HCV) infection provides an important basis for the identification and diagnosis of patients with HCV infection. With the continuous development of HCV testing in recent years, the performance of reagents has been significantly improved, and new testing service strategies have emerged and gradually been applied in clinical practice. This article summarizes the laboratory testing methods and strategies for HCV infection in China and globally, as well as the testing methods for HCV infection, and analyzes the influence of new methods and strategies on the prevention and control of HCV infection in China. Timely and accurate laboratory testing methods and effective and feasible testing strategies may help to realize the early identification, early diagnosis, and early treatment of HCV infection and ultimately achieve the strategic goal of eliminating viral hepatitis as a major public health threat by 2030.
-
Key words:
- Hepacivirus /
- Biomarkers /
- Diagnosis
-
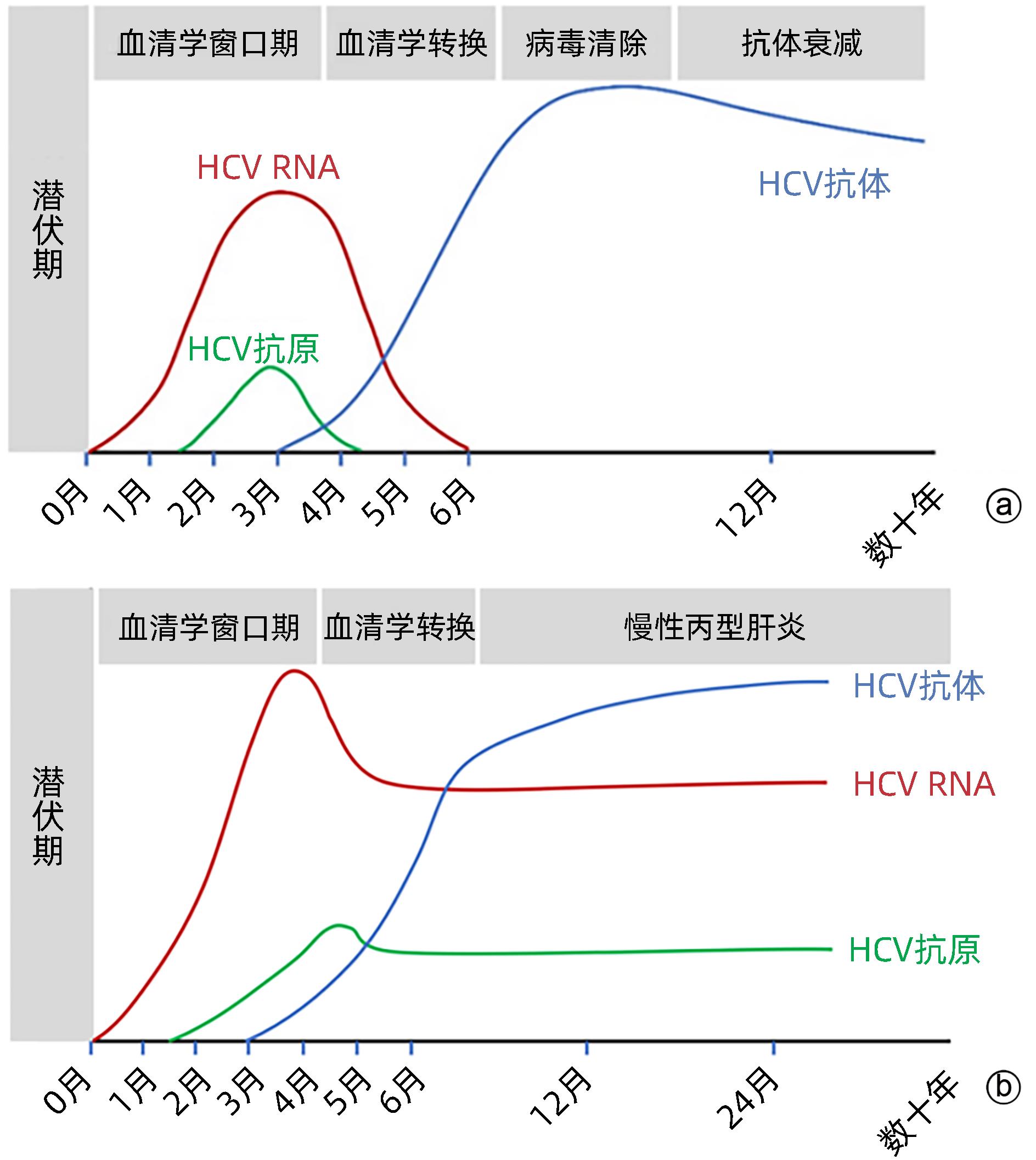
注: a,自限性HCV感染;b,慢性HCV感染。
图 1 外周血中HCV感染检测标志物的动态变化[5]
Figure 1. Dynamic change of biomarkers for HCV testing in peripheral blood
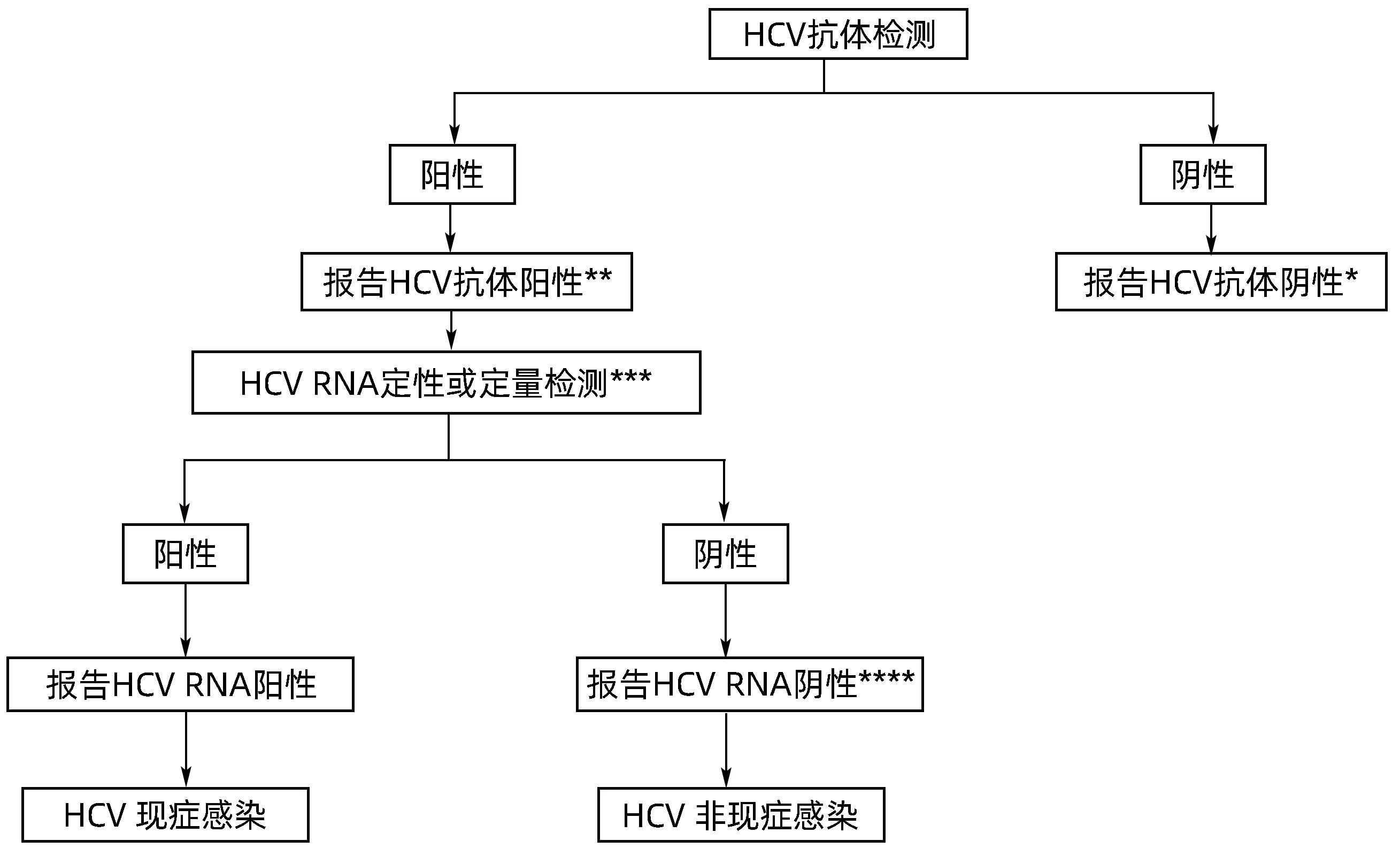
表 1 获批的HCV感染检测方法和检测产品数量
Table 1. Approved HCV testing methods and products
检测方法 检测产品的数量 总计 国产产品 进口产品 ELISA 抗体 25 23 2 抗原 4 4 0 抗原抗体 2 2 0 CLIA 抗体 37 31 6 抗原 2 1 1 抗原抗体 1 1 0 快检(抗体) 29 29 0 WB(抗体) 1 0 1 RIBA(抗体) 2 1 1 核酸检测 RNA定性 4 1 3 RNA定量 31 25 6 RNA定性+定量 4 0 4 表 2 HCV筛查试剂的发展
Table 2. Development of HCV screening reagents
项目 第一代 第二代 第三代 第四代 靶点 NS4(c100-3) C,NS3,NS4 C,NS3,NS4 衣壳抗原,C,NS3,NS4 敏感度 <95% >95% >95% >99% 特异度 <95% >95% >95% >99% 窗口期 16~24周 7~8周 7~8周 4~8周 -
[1] MARTINELLO M, SOLOMON SS, TERRAULT NA, et al. Hepatitis C[J]. Lancet, 2023, 402( 10407): 1085- 1096. DOI: 10.1016/s0140-6736(23)01320-x. [2] World Health Organization. Hepatitis C[EB/OL].( 2023-07-18)[ 2023-11-22]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c [3] World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021[M]. Geneva: WHO, 2016. [4] World Health Organization. Guidelines on Hepatitis B and C Testing[M]. Geneva: WHO, 2017. [5] Chinese Centers for Disease Control and Prevention. Technical Specification for Laboratory Testing of Hepatitis C Virus(2023 Revised Edition)[Z]. 2023.中国疾病预防控制中心. 丙型肝炎病毒实验室检测技术规范(2023年修订版)[Z]. 2023. [6] National Health and Family Planning Commission. Diagnosis for hepatitis C: WS 213—2018[S]. Beijing: Standards Press of China, 2018: 1- 9.国家卫生和计划生育委员会. 丙型肝炎诊断: WS 213—2018[S]. 北京: 中国标准出版社, 2018: 1- 9. [7] European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series[J]. J Hepatol, 2020, 73( 5): 1170- 1218. DOI: 10.1016/j.jhep.2020.08.018. [8] WARKAD SD, SONG KS, PAL D, et al. Developments in the HCV screening technologies based on the detection of antigens and antibodies[J]. Sensors, 2019, 19( 19): 4257. DOI: 10.3390/s19194257. [9] GUPTA E, BAJPAI M, CHOUDHARY A. Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays[J]. Asian J Transfus Sci, 2014, 8( 1): 19- 25. DOI: 10.4103/0973-6247.126683. [10] DUFOUR DR, TALASTAS M, FERNANDEZ MDA, et al. Chemiluminescence assay improves specificity of hepatitis C antibody detection[J]. Clin Chem, 2003, 49( 6 Pt 1): 940- 944. DOI: 10.1373/49.6.940. [11] SMITH BD, JEWETT A, DROBENIUC J, et al. Rapid diagnostic HCV antibody assays[J]. Antivir Ther, 2012, 17( 7 Pt B): 1409- 1413. DOI: 10.3851/IMP2470. [12] Centers for Disease Control and Prevention(CDC). Testing for HCV infection: An update of guidance for clinicians and laboratorians[J]. MMWR Morb Mortal Wkly Rep, 2013, 62( 18): 362- 365. [13] WARKAD SD, NIMSE SB, SONG KS, et al. HCV detection, discrimination, and genotyping technologies[J]. Sensors, 2018, 18( 10): 3423. DOI: 10.3390/s18103423. [14] SCHALASTA G, SPEICHER A, BÖRNER A, et al. Performance of the new aptima HCV quant dx assay in comparison to the cobas TaqMan HCV2 test for use with the high pure system in detection and quantification of hepatitis C virus RNA in plasma or serum[J]. J Clin Microbiol, 2016, 54( 4): 1101- 1107. DOI: 10.1128/JCM.03236-15. [15] HENDRICKS DA, FRIESENHAHN M, TANIMOTO L, et al. Multicenter evaluation of the versant hcv RNA qualitative assay for detection of hepatitis c virus RNA[J]. J Clin Microbiol, 2003, 41( 2): 651- 656. DOI: 10.1128/JCM.41.2.651-656.2003. [16] SCOTT JD, GRETCH DR. Molecular diagnostics of hepatitis C virus infection: A systematic review[J]. JAMA, 2007, 297( 7): 724- 732. DOI: 10.1001/jama.297.7.724. [17] CATLETT B, CARRERA A, STARR M, et al. Performance evaluation of the Hologic Aptima HCV Quant Dx assay for detection of HCV RNA from dried blood spots[J]. J Clin Virol, 2019, 112: 40- 44. DOI: 10.1016/j.jcv.2019.01.010. [18] WEBER J, SAHOO MK, TAYLOR N, et al. Evaluation of the aptima HCV quant dx assay using serum and dried blood spots[J]. J Clin Microbiol, 2019, 57( 4): e00030-19. DOI: 10.1128/JCM.00030-19. [19] ROSS RS, VIAZOV S, SALLOUM S, et al. Analytical performance characteristics and clinical utility of a novel assay for total hepatitis C virus core antigen quantification[J]. J Clin Microbiol, 2010, 48( 4): 1161- 1168. DOI: 10.1128/JCM.01640-09. [20] FREIMAN JM, TRAN TM, SCHUMACHER SG, et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: A systematic review and meta-analysis[J]. Ann Intern Med, 2016, 165( 5): 345- 355. DOI: 10.7326/M16-0065. [21] LAMOURY FMJ, SOKER A, MARTINEZ D, et al. Hepatitis C virus core antigen: A simplified treatment monitoring tool, including for post-treatment relapse[J]. J Clin Virol, 2017, 92: 32- 38. DOI: 10.1016/j.jcv.2017.05.007. [22] CHEVALIEZ S, FELD J, CHENG K, et al. Clinical utility of HCV core antigen detection and quantification in the diagnosis and management of patients with chronic hepatitis C receiving an all-oral, interferon-free regimen[J]. Antivir Ther, 2018, 23( 3): 211- 217. DOI: 10.3851/IMP3042. [23] VAN TILBORG M, MARZOOQI SH AL, WONG WWL, et al. HCV core antigen as an alternative to HCV RNA testing in the era of direct-acting antivirals: Retrospective screening and diagnostic cohort studies[J]. Lancet Gastroenterol Hepatol, 2018, 3( 12): 856- 864. DOI: 10.1016/S2468-1253(18)30271-1. [24] KUMAR R, CHAN KP, EKSTROM VSM, et al. Hepatitis C virus antigen detection is an appropriate test for screening and early diagnosis of hepatitis C virus infection in at-risk populations and immunocompromised hosts[J]. J Med Virol, 2021, 93( 6): 3738- 3743. DOI: 10.1002/jmv.26433. [25] HONGJAISEE S, DOUNGJINDA N, KHAMDUANG W, et al. Rapid visual detection of hepatitis C virus using a reverse transcription loop-mediated isothermal amplification assay[J]. Int J Infect Dis, 2021, 102: 440- 445. DOI: 10.1016/j.ijid.2020.10.082. [26] CHIA CT, BENDER AT, LILLIS L, et al. Rapid detection of hepatitis C virus using recombinase polymerase amplification[J]. PLoS One, 2022, 17( 10): e0276582. DOI: 10.1371/journal.pone.0276582. [27] VIDIMLISKI PD, NIKOLOV I, GESHKOVSKA NM, et al. Review: Occult hepatitis C virus infection: Still remains a controversy[J]. J Med Virol, 2014, 86( 9): 1491- 1498. DOI: 10.1002/jmv.23979. [28] KHATTAB MA, ZAKARIA Y, SADEK E, et al. Detection of hepatitis C virus(HCV) RNA in the peripheral blood mononuclear cells of HCV-infected patients following sustained virologic response[J]. Clin Exp Med, 2023, 23( 1): 131- 140. DOI: 10.1007/s10238-022-00791-7. [29] FRÍAS M, RIVERO-JUÁREZ A, TÉLLEZ F, et al. Evaluation of hepatitis C viral RNA persistence in HIV-infected patients with long-term sustained virological response by droplet digital PCR[J]. Sci Rep, 2019, 9( 1): 12507. DOI: 10.1038/s41598-019-48966-9. [30] World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection[M]. Geneva: WHO, 2016. [31] WORKOWSKI KA, BACHMANN LH, CHAN PA, et al. Sexually transmitted infections treatment guidelines, 2021[J]. MMWR Recomm Rep, 2021, 70( 4): 1- 187. DOI: 10.15585/mmwr.rr7004a1. [32] CHEVALIEZ S, PAWLOTSKY JM. New virological tools for screening, diagnosis and monitoring of hepatitis B and C in resource-limited settings[J]. J Hepatol, 2018, 69( 4): 916- 926. DOI: 10.1016/j.jhep.2018.05.017. [33] World Health Organization. Updated recommendations on simplified service delivery and diagnostics for hepatitis C infection[M]. Geneva: WHO, 2022. [34] GREBELY J, LAMOURY FMJ, HAJARIZADEH B, et al. Evaluation of the Xpert HCV Viral Load point-of-care assay from venepuncture-collected and finger-stick capillary whole-blood samples: A cohort study[J]. Lancet Gastroenterol Hepatol, 2017, 2( 7): 514- 520. DOI: 10.1016/S2468-1253(17)30075-4. [35] TRICKEY A, FAJARDO E, ALEMU D, et al. Impact of hepatitis C virus point-of-care RNA viral load testing compared with laboratory-based testing on uptake of RNA testing and treatment, and turnaround times: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2023, 8( 3): 253- 270. DOI: 10.1016/S2468-1253(22)00346-6. [36] ORU E, TRICKEY A, SHIRALI R, et al. Decentralisation, integration, and task-shifting in hepatitis C virus infection testing and treatment: A global systematic review and meta-analysis[J]. Lancet Glob Health, 2021, 9( 4): e431- e445. DOI: 10.1016/S2214-109X(20)30505-2. [37] TAO YS, TANG WM, FAJARDO E, et al. Reflex hepatitis C virus viral load testing following an initial positive hepatitis C virus antibody test: A global systematic review and meta-analysis[J]. Clin Infect Dis, 2023, 77( 8): 1137- 1156. DOI: 10.1093/cid/ciad126. [38] THOMPSON LA, FENTON J, CHARLTON CL. HCV reflex testing: A single-sample, low-contamination method that improves the diagnostic efficiency of HCV testing among patients in Alberta, Canada[J]. J Assoc Med Microbiol Infect Dis Can, 2022, 7( 2): 97- 107. DOI: 10.3138/jammi-2021-0028. [39] MANTEUFFEL JJ, LEE MS, BUSSA RM, et al. Hepatitis C virus reflex testing protocol in an emergency department[J]. West J Emerg Med, 2022, 23( 2): 108- 114. DOI: 10.5811/westjem.2021.10.52468. [40] DE LA PAZ CASAS M, GARCÍA F, FREYRE-CARRILLO C, et al. Towards the elimination of hepatitis C: Implementation of reflex testing in Andalusia[J]. Rev Esp Enferm Dig, 2020, 112( 7): 515- 519. DOI: 10.17235/reed.2020.6370/2019. [41] JOHNSON LN, GAYNOR AM, WROBLEWSKI K, et al. Maximizing reflexive HCV RNA testing of HCV antibody-reactive samples within United States public health laboratories[J]. J Infect Dis, 2023: jiad191. DOI: 10.1093/infdis/jiad191. [42] World Health Organization. Recommendations and guidance on hepatitis C virus self-testing[M]. Geneva: WHO, 2021. -



 PDF下载 ( 928 KB)
PDF下载 ( 928 KB)

 下载:
下载: