CD8+T淋巴细胞在非酒精性脂肪性肝炎中的作用
DOI: 10.12449/JCH241026
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:赵一鸣负责设计论文框架及撰写论文;李开楊负责指导撰写文章并最后定稿;杨梅负责论文修改;赵琦负责指导撰写文章。
-
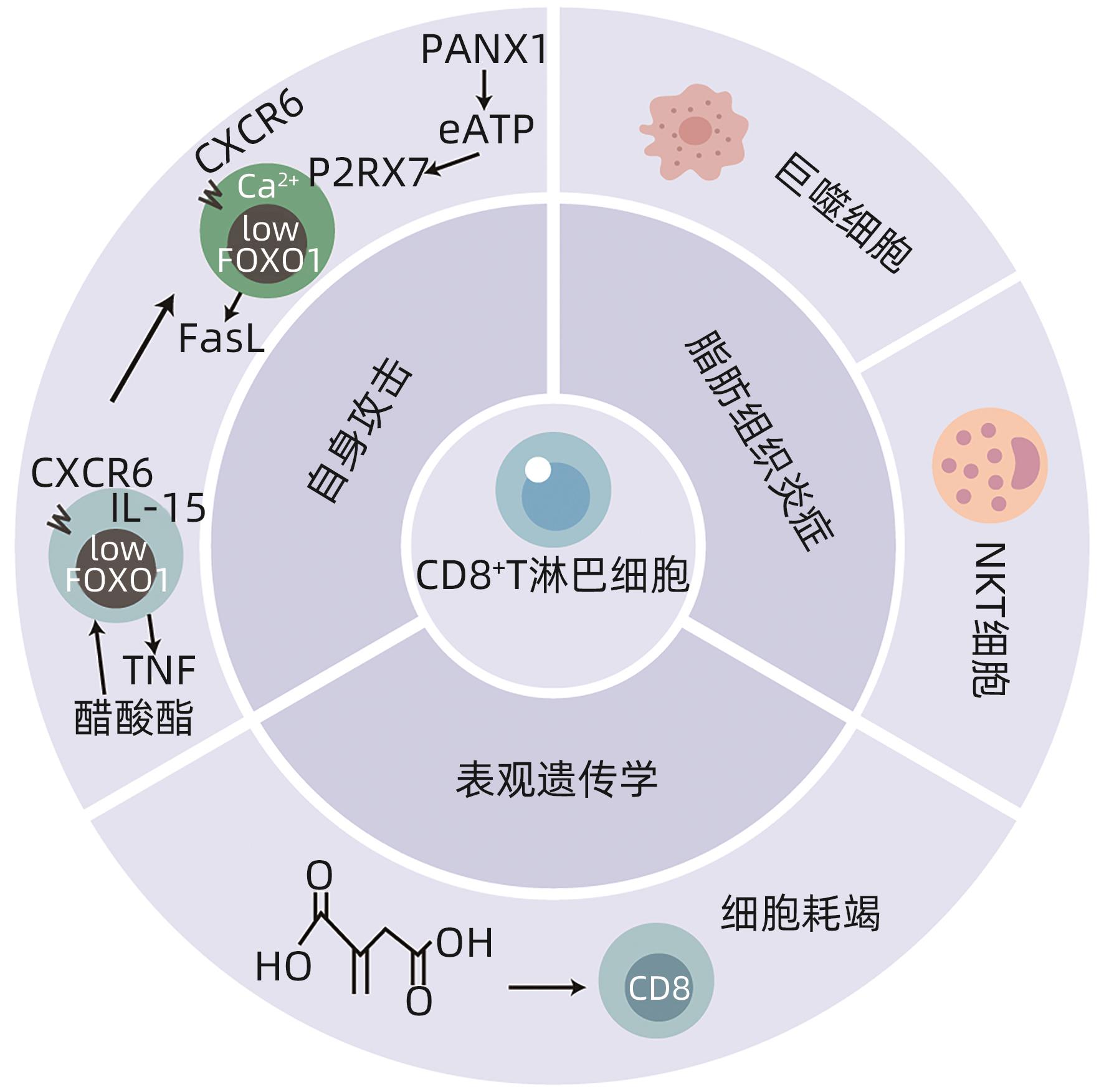
摘要: 非酒精性脂肪性肝炎(NASH)是一种与代谢综合征密切相关的肝脏疾病,其发病机制包括胰岛素抵抗、脂质代谢紊乱、炎症反应等方面。本文简述了CD8+T淋巴细胞通过Fas-Fas配体(FasL)信号通路、释放穿孔素和颗粒酶以及分泌肿瘤坏死因子(TNF)α等细胞因子三种途径发挥细胞毒性作用特异性杀伤病毒及肿瘤靶细胞参与免疫调节;同时总结了CD8+T淋巴细胞促进巨噬细胞招募且与自然杀伤T淋巴细胞协同参与脂肪组织炎症发展,并通过自身攻击及表观遗传学机制导致肝损伤,促进NASH发病。由此得知CD8+T淋巴细胞可能成为NASH新的治疗靶点,未来继续深入研究CD8+T淋巴细胞在NASH中影响发病进程的具体机制有利于进一步指导临床治疗。
-
关键词:
- 非酒精性脂肪性肝病 /
- CD8阳性T淋巴细胞 /
- 脂肪组织 /
- 炎症 /
- 表观基因组学
Abstract: Nonalcoholic steatohepatitis (NASH) is a liver disease closely associated with metabolic syndrome, and its pathogenesis includes insulin resistance, lipid metabolism disorders, and inflammatory response. This article briefly describes how CD8+ T cells exert a cytotoxic effect through the three pathways of the Fas-Fas ligand signaling pathway, the release of perforin and granzymes, and the secretion of tumor necrosis factor-α and other cytokines, and these mechanisms allow CD8+ T cells to specifically kill virus-infected cells and tumor target cells, thereby participating in immune regulation. At the same time, this article summarizes that CD8+ T cells promote the recruitment of macrophages cooperate with natural killer T cells to participate in the development of adipose tissue inflammation, and lead to liver injury through automatic attack and epigenetic mechanisms, finally promoting the pathogenesis of NASH. It is concluded that CD8+ T cells may become a new therapeutic target for NASH, and in-depth research on the specific mechanism of CD8+ T cells affecting the pathogenesis in NASH in the future may help to further guide clinical treatment. -
[1] PAIK JM, GOLABI P, YOUNOSSI Y, et al. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD[J]. Hepatology, 2020, 72( 5): 1605- 1616. DOI: 10.1002/hep.31173. [2] NOUREDDIN M, VIPANI A, BRESEE C, et al. NASH leading cause of liver transplant in women: Updated analysis of indications for liver transplant and ethnic and gender variances[J]. Am J Gastroenterol, 2018, 113( 11): 1649- 1659. DOI: 10.1038/s41395-018-0088-6. [3] LI J, ZOU BY, YEO YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2019, 4( 5): 389- 398. DOI: 10.1016/S2468-1253(19)30039-1. [4] YOUNOSSI Z, ANSTEE QM, MARIETTI M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention[J]. Nat Rev Gastroenterol Hepatol, 2018, 15( 1): 11- 20. DOI: 10.1038/nrgastro.2017.109. [5] SHEKA AC, ADEYI O, THOMPSON J, et al. Nonalcoholic steatohepatitis: A review[J]. JAMA, 2020, 323( 12): 1175- 1183. DOI: 10.1001/jama.2020.2298. [6] ZHAO YC, GAO LC, JIANG CL, et al. The transcription factor zinc fingers and homeoboxes 2 alleviates NASH by transcriptional activation of phosphatase and tensin homolog[J]. Hepatology, 2022, 75( 4): 939- 954. DOI: 10.1002/hep.32165. [7] LENG YR, ZHANG MH, LUO JG, et al. Pathogenesis of NASH and promising natural products[J]. Chin J Nat Med, 2021, 19( 1): 12- 27. DOI: 10.1016/S1875-5364(21)60002-X. [8] FRIEDMAN SL, NEUSCHWANDER-TETRI BA, RINELLA M, et al. Mechanisms of NAFLD development and therapeutic strategies[J]. Nat Med, 2018, 24( 7): 908- 922. DOI: 10.1038/s41591-018-0104-9. [9] SUTTI S, ALBANO E. Adaptive immunity: An emerging player in the progression of NAFLD[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 2): 81- 92. DOI: 10.1038/s41575-019-0210-2. [10] PROKHNEVSKA N, CARDENAS MA, VALANPARAMBIL RM, et al. CD8+ T cell activation in cancer comprises an initial activation phase in lymph nodes followed by effector differentiation within the tumor[J]. Immunity, 2023, 56( 1): 107- 124.e5. DOI: 10.1016/j.immuni.2022.12.002. [11] SUN QL, CAI DL, LIU DF, et al. BCL6 promotes a stem-like CD8+ T cell program in cancer via antagonizing BLIMP1[J]. Sci Immunol, 2023, 8( 88): eadh1306. DOI: 10.1126/sciimmunol.adh1306. [12] SUN LN, SU YH, JIAO AJ, et al. T cells in health and disease[J]. Signal Transduct Target Ther, 2023, 8( 1): 235. DOI: 10.1038/s41392-023-01471-y. [13] BAI L, XIN YJ, DUAN DY, et al. Advances in macrophage function and its anti-inflammatory and proresolving activity and role in periodontitis development[J]. West China J Stomatol, 2017, 35( 4): 427- 432. DOI: 10.7518/hxkq.2017.04.016. [14] AICHELE P, NEUMANN-HAEFELIN C, EHL S, et al. Immunopathology caused by impaired CD8+ T-cell responses[J]. Eur J Immunol, 2022, 52( 9): 1390- 1395. DOI: 10.1002/eji.202149528. [15] RITTER AT, SHTENGEL G, XU CS, et al. ESCRT-mediated membrane repair protects tumor-derived cells against T cell attack[J]. Science, 2022, 376( 6591): 377- 382. DOI: 10.1126/science.abl3855. [16] ZHAO WJ, ZHU Y, LU ZX. Radix tetrastigme polysaccharide promotes antitumor immune response in lewis lung cancer mice[J]. Chin J Lung Cancer, 2023, 26( 8): 559- 571. DOI: 10.3779/j.issn.1009-3419.2023.106.16.赵文菊, 朱勇, 鲁正学. 三叶青多糖增强Lewis肺癌小鼠抗肿瘤免疫反应[J]. 中国肺癌杂志, 2023, 26( 8): 559- 571. DOI: 10.3779/j.issn.1009-3419.2023.106.16. [17] GLUKHOVA XA, TRIZNA JA, PROUSSAKOVA OV, et al. Impairment of Fas-ligand-caveolin-1 interaction inhibits Fas-ligand translocation to rafts and Fas-ligand-induced cell death[J]. Cell Death Dis, 2018, 9( 2): 73. DOI: 10.1038/s41419-017-0109-1. [18] WILSON NS, DIXIT V, ASHKENAZI A. Death receptor signal transducers: Nodes of coordination in immune signaling networks[J]. Nat Immunol, 2009, 10( 4): 348- 355. DOI: 10.1038/ni.1714. [19] POBEZINSKAYA YL, LIU ZG. The role of TRADD in death receptor signaling[J]. Cell Cycle, 2012, 11( 5): 871- 876. DOI: 10.4161/cc.11.5.19300. [20] HOLBROOK J, LARA-REYNA S, JAROSZ-GRIFFITHS H, et al. Tumour necrosis factor signalling in health and disease[J]. F1000Res, 2019, 8: F1000 Faculty Rev-111. DOI: 10.12688/f1000research.17023.1. [21] CHADWICK W, MAGNUS T, MARTIN B, et al. Targeting TNF-alpha receptors for neurotherapeutics[J]. Trends Neurosci, 2008, 31( 10): 504- 511. DOI: 10.1016/j.tins.2008.07.005. [22] HAMMERICH L, TACKE F. Hepatic inflammatory responses in liver fibrosis[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 10): 633- 646. DOI: 10.1038/s41575-023-00807-x. [23] DUAN HX, JING L, XIANG JQ, et al. CD146 associates with Gp130 to control a macrophage pro-inflammatory program that regulates the metabolic response to obesity[J]. Adv Sci, 2022, 9( 13): e2103719. DOI: 10.1002/advs.202103719. [24] NISHIMURA S, MANABE I, NAGASAKI M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity[J]. Nat Med, 2009, 15( 8): 914- 920. DOI: 10.1038/nm.1964. [25] DUFFAUT C, GALITZKY J, LAFONTAN M, et al. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity[J]. Biochem Biophys Res Commun, 2009, 384( 4): 482- 485. DOI: 10.1016/j.bbrc.2009.05.002. [26] KIRAN S, KUMAR V, MURPHY EA, et al. High fat diet-induced CD8+ T cells in adipose tissue mediate macrophages to sustain low-grade chronic inflammation[J]. Front Immunol, 2021, 12: 680944. DOI: 10.3389/fimmu.2021.680944. [27] RUF B, GRETEN TF, KORANGY F. Innate lymphoid cells and innate-like T cells in cancer- at the crossroads of innate and adaptive immunity[J]. Nat Rev Cancer, 2023, 23( 6): 351- 371. DOI: 10.1038/s41568-023-00562-w. [28] MARICIC I, MARRERO I, EGUCHI A, et al. Differential activation of hepatic invariant NKT cell subsets plays a key role in progression of nonalcoholic steatohepatitis[J]. J Immunol, 2018, 201( 10): 3017- 3035. DOI: 10.4049/jimmunol.1800614. [29] BHATTACHARJEE J, KIRBY M, SOFTIC S, et al. Hepatic natural killer T-cell and CD8+ T-cell signatures in mice with nonalcoholic steatohepatitis[J]. Hepatol Commun, 2017, 1( 4): 299- 310. DOI: 10.1002/hep4.1041. [30] DENG T, LYON CJ, MINZE LJ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation[J]. Cell Metab, 2013, 17( 3): 411- 422. DOI: 10.1016/j.cmet.2013.02.009. [31] FERNANDEZ-RUIZ D, NG WY, HOLZ LE, et al. Liver-resident memory CD8+ T cells form a front-line defense against malaria liver-stage infection[J]. Immunity, 2019, 51( 4): 780. DOI: 10.1016/j.immuni.2019.09.019. [32] TOPHAM DJ, REILLY EC. Tissue-resident memory CD8+ T cells: From phenotype to function[J]. Front Immunol, 2018, 9: 515. DOI: 10.3389/fimmu.2018.00515. [33] KHAN O, GILES JR, MCDONALD S, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion[J]. Nature, 2019, 571( 7764): 211- 218. DOI: 10.1038/s41586-019-1325-x. [34] ALFEI F, KANEV K, HOFMANN M, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection[J]. Nature, 2019, 571( 7764): 265- 269. DOI: 10.1038/s41586-019-1326-9. [35] LEONARD WJ, LIN JX, O’SHEA JJ. The γc family of cytokines: Basic biology to therapeutic ramifications[J]. Immunity, 2019, 50( 4): 832- 850. DOI: 10.1016/j.immuni.2019.03.028. [36] DUDEK M, PFISTER D, DONAKONDA S, et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH[J]. Nature, 2021, 592( 7854): 444- 449. DOI: 10.1038/s41586-021-03233-8. [37] LINDEN J, KOCH-NOLTE F, DAHL G. Purine release, metabolism, and signaling in the inflammatory response[J]. Annu Rev Immunol, 2019, 37: 325- 347. DOI: 10.1146/annurev-immunol-051116-052406. [38] LI YY. Modern epigenetics methods in biological research[J]. Methods, 2021, 187: 104- 113. DOI: 10.1016/j.ymeth.2020.06.022. [39] LUO CY, HAJKOVA P, ECKER JR. Dynamic DNA methylation: In the right place at the right time[J]. Science, 2018, 361( 6409): 1336- 1340. DOI: 10.1126/science.aat6806. [40] PANG BR, SUI SY, WANG Q, et al. Upregulation of DLEU1 expression by epigenetic modification promotes tumorigenesis in human cancer[J]. J Cell Physiol, 2019, 234( 10): 17420- 17432. DOI: 10.1002/jcp.28364. [41] RECILLAS-TARGA F. Cancer epigenetics: An overview[J]. Arch Med Res, 2022, 53( 8): 732- 740. DOI: 10.1016/j.arcmed.2022.11.003. [42] STEPANOV AI, BESEDOVSKAIA ZV, MOSHAREVA MA, et al. Studying chromatin epigenetics with fluorescence microscopy[J]. Int J Mol Sci, 2022, 23( 16): 8988. DOI: 10.3390/ijms23168988. [43] KIMURA M, IGUCHI T, IWASAWA K, et al. En masse organoid phenotyping informs metabolic-associated genetic susceptibility to NASH[J]. Cell, 2022, 185( 22): 4216- 4232. e 16. DOI: 10.1016/j.cell.2022.09.031. [44] FORD BR, POHOLEK AC. Regulation and immunotherapeutic targeting of the epigenome in exhausted CD8 T cell responses[J]. J Immunol, 2023, 210( 7): 869- 879. DOI: 10.4049/jimmunol.2200681. [45] ZHAO HY, TENG D, YANG LF, et al. Myeloid-derived itaconate suppresses cytotoxic CD8+ T cells and promotes tumour growth[J]. Nat Metab, 2022, 4( 12): 1660- 1673. DOI: 10.1038/s42255-022-00676-9. [46] GLOBIG AM, ZHAO S, ROGINSKY J, et al. The β1-adrenergic receptor links sympathetic nerves to T cell exhaustion[J]. Nature, 2023, 622( 7982): 383- 392. DOI: 10.1038/s41586-023-06568-6. -



 PDF下载 ( 842 KB)
PDF下载 ( 842 KB)

 下载:
下载:


