中性粒细胞胞外诱捕网在原发性胆汁性胆管炎患者中的作用初步探析
DOI: 10.3969/j.issn.1001-5256.2022.04.014
A preliminary study of the role of neutrophil extracellular traps in patients with primary biliary cholangitis
-
摘要:
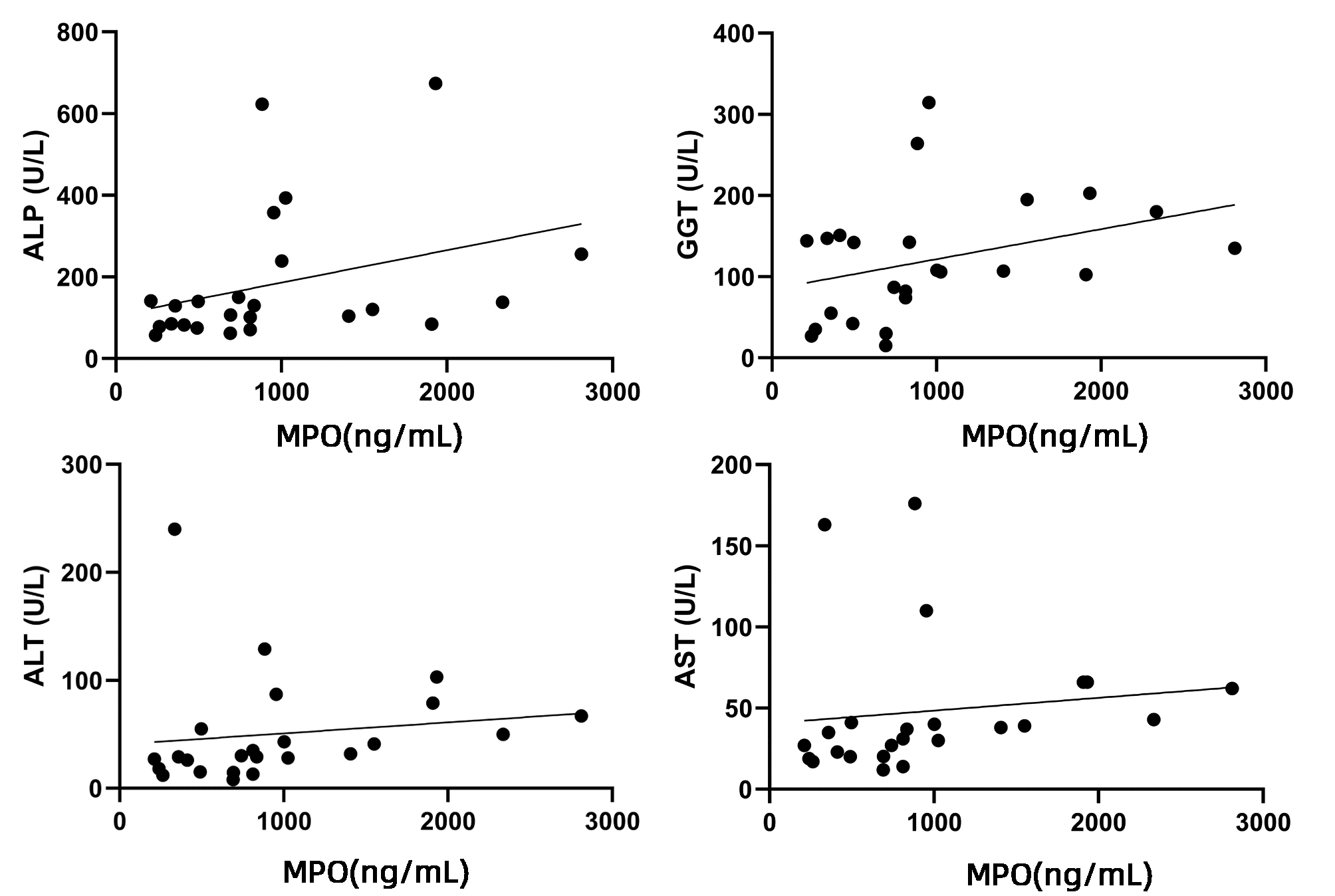
目的 探索原发性胆汁性胆管炎(PBC)患者外周血和肝组织内中性粒细胞胞外诱捕网(NET)表达水平,及其与临床生化指标的相关性。 方法 选取2016年8月—2020年8月上海交通大学医学院附属仁济医院收治的PBC患者24例、年龄匹配的原发性硬化性胆管炎(PSC)8例以及自身免疫性肝炎(AIH)患者19例、健康对照(HC)18例,分别检测血清中髓过氧化物酶(MPO)水平,并与临床指标进行相关性分析。利用组织免疫荧光检测PBC患者肝内NET表达情况。体外实验比较PBC患者和健康对照组外周血中性粒细胞产生NET能力的差异。满足正态分布的计量资料2组间比较采用独立样本t检验,非正态分布计量资料多组间比较采用Kruskal-Wallis H检验,2组间比较采用Mann-Whitney U检验。对MPO水平与肝脏相关实验室指标进行相关性分析,计算Spearman相关系数。 结果 PBC组血清中的MPO水平[811.21(450.67~1 216.20)ng/mL]较AIH组[468.58(142.63~812.43)ng/mL]和HC组[357.54(203.52~811.21)ng/mL] 明显升高(P值均<0.05);提示在PBC患者外周血中NET的产生量显著增加。PSC患者血清中MPO水平[763.56(489.59~1 633.14)ng/mL]也较HC组显著升高(P<0.05)。MPO水平与ALP(r=0.500,P<0.05)、GGT(r=0.426,P<0.05)、ALT(r=0.521,P<0.01)和AST(r=0.547,P<0.01)水平之间存在正相关关系。免疫荧光共聚焦显示PBC患者肝内存在H3Cit、MPO共定位。体外实验观察到PBC患者外周血中性粒细胞较健康对照在体外刺激时产生NET增多,且自发产生NET亦增高。 结论 PBC患者外周血及肝内NET增多,且外周血NET的含量与肝功能生化指标呈正相关。NET或可成为评估疾病严重程度的新型生物学标志物。 Abstract:Objective To investigate the expression level of neutrophil extracellular traps (NET) in the peripheral blood and liver tissue of primary biliary cholangitis (PBC) patients and its correlation with clinical biochemical parameters. Methods A total of 24 PBC patients who were admitted to Renji Hospital, Shanghai Jiao Tong University School of Medicine, from August 2016 to August 2020 were enrolled, as well as 8 patients with primary sclerosing cholangitis (PSC) and 19 patients with autoimmune hepatitis (AIH) matched for age, and 19 healthy individuals were enrolled as healthy control group (HC group). The serum level of myeloperoxidase (MPO) was measured, and its correlation with clinical indices were analyzed. Immunofluorescence assay was used to measure the expression of NET in the liver of PBC patients, and an in vitro experiment was to compare the ability of peripheral blood neutrophils to produce NET between PBC patients and healthy controls. Normally distributed continuous data were expressed as mean±standard deviation, and the independent samples t-test was used for comparison between two groups; for the non-normally distributed continuous data expressed as M(P25-P75), the Kruskal-Wallis H test was used for comparison between multiple groups, and the Mann-Whitney U test was used for comparison between two groups. A correlation analysis was performed for MPO level and liver-related laboratory markers, and the Spearman's correlation coefficient was calculated. Results The serum level of MPO in the PBC group was increased to 811.21 (450.67-1 216.20) ng/mL, which was significantly higher than that in the AIH group [468.58 (142.63-812.43) ng/mL] and the HC group [357.54 (203.52-811.21) ng/mL] (P < 0.05), suggesting that there was a significant increase in the production of NET in peripheral blood of PBC patients. The PSC patients had a serum MPO level of 763.56 (489.59-1 633.14) ng/mL, which was significantly higher than that in the HC group (P < 0.05). MPO level was positively correlated with alkaline phosphatase (r=0.500, P < 0.05), gamma-glutamyl transpeptidase (r=0.426, P < 0.05), alanine aminotransferase (r=0.521, P < 0.05), and aspartate aminotransferase (r=0.547, P < 0.01). Confocal immunofluorescence showed colocalization of H3Cit and MPO in the liver of PBC patients. In vitro experiment showed that compared with the HC group, the PBC group had an increase in NET produced by peripheral blood neutrophils after in vitro stimulation and an increase in spontaneous production of NET. Conclusion There is an increase in NET in peripheral blood and liver of PBC patients, and the content of peripheral blood NET is positively correlated with biochemical parameters of liver function. NET may become a novel biomarker for assessing the severity of PBC. -
Key words:
- Primary Biliary Cholangitis /
- Neutrophils /
- Extracellular Traps
-
注:a,4组血清中MPO水平;b,PBC患者血清线粒体抗体(AMA)阳性或阴性者MPO水平;c,PBC患者血清抗2-丙酮酸脱氢酶(M2-3E)抗体阳性或阴性者MPO水平;d,PBC患者血清抗线粒体-M2型抗体(M2)阳性或阴性者MPO水平;e,PBC患者血清抗斑点蛋白抗体(sp100)阳性或阴性者MPO水平;f,PBC患者血清抗糖蛋白210抗体(gp210)阳性或阴性者MPO水平。
图 1 外周血MPO水平与PBC相关自身抗体的相关性
Figure 1. Correlation analysis between peripheral blood MPO levels and autoantibodies of PBC
表 1 PBC患者肝脏生化指标基本资料
Table 1. Basic information of liver biochemical indicators in PBC patients
指标 数值 与MPO相关性 r值 P值 ALP (U/L) 124.50(83.00~194.50) 0.500 0.013 GGT (U/L) 107.50(64.50~149.00) 0.426 0.038 ALT (U/L) 31.00(22.10~61.00) 0.521 0.009 AST (U/L) 36.00(21.65~52.50) 0.547 0.006 TBil (μmol/L) 12.95(8.95~14.90) 0.380 0.067 DBil (μmol/L) 4.40(3.15~6.35) 0.267 0.206 IgM (g/L) 2.87(1.98~3.91) 0.007 0.976 -
[1] LI B, ZHANG J, CHEN Y, et al. Alterations in microbiota and their metabolites are associated with beneficial effects of bile acid sequestrant on icteric primary biliary cholangitis[J]. Gut Microbes, 2021, 13(1): 1946366. DOI: 10.1080/19490976.2021.1946366. [2] WANG C, ZHENG X, JIANG P, et al. Genome-wide association studies of specific antinuclear autoantibody subphenotypes in primary biliary cholangitis[J]. Hepatology, 2019, 70(1): 294-307. DOI: 10.1002/hep.30604. [3] HU ML, WANG QX, MA X. Considerations on the elevated level of aminotransferase in primary biliary cholangitis[J]. J Clin Hepatol, 2021, 37(10): 2277-2279. DOI: 10.3969/j.issn.1001-5256.2021.10.005.胡明礼, 王绮夏, 马雄. 原发性胆汁性胆管炎合并转氨酶升高的思考[J]. 临床肝胆病杂志, 2021, 37(10): 2277-2279. DOI: 10.3969/j.issn.1001-5256.2021.10.005. [4] QIU F, TANG R, ZUO X, et al. A genome-wide association study identifies six novel risk loci for primary biliary cholangitis[J]. Nat Commun, 2017, 8: 14828. DOI: 10.1038/ncomms14828. [5] FONSECA Z, DÍAZ-GODÍNEZ C, MORA N, et al. Entamoeba histolytica induce signaling via Raf/MEK/ERK for neutrophil extracellular trap (NET) formation[J]. Front Cell Infect Microbiol, 2018, 8: 226. DOI: 10.3389/fcimb.2018.00226. [6] KESSENBROCK K, KRUMBHOLZ M, SCHÖNERMARCK U, et al. Netting neutrophils in autoimmune small-vessel vasculitis[J]. Nat Med, 2009, 15(6): 623-625. DOI: 10.1038/nm.1959. [7] WRIGHT HL, LYON M, CHAPMAN EA, et al. Rheumatoid arthritis synovial fluid neutrophils drive inflammation through production of chemokines, reactive oxygen species, and neutrophil extracellular traps[J]. Front Immunol, 2020, 11: 584116. DOI: 10.3389/fimmu.2020.584116. [8] TAY SH, CELHAR T, FAIRHURST AM. Low-density neutrophils in systemic lupus erythematosus[J]. Arthritis Rheumatol, 2020, 72(10): 1587-1595. DOI: 10.1002/art.41395. [9] DOS SANTOS RAMOS A, VIANA G, DE MACEDO BRIGIDO M, et al. Neutrophil extracellular traps in inflammatory bowel diseases: Implications in pathogenesis and therapeutic targets[J]. Pharmacol Res, 2021, 171: 105779. DOI: 10.1016/j.phrs.2021.105779. [10] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and management of primary biliary cirrhosis (cholangitis)(2015)[J]. J Clin Hepatol, 2015, 31(12): 1980-1988. DOI: 10.3969/j.issn.1001-5256.2015.12.004.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 原发性胆汁性肝硬化(又名原发性胆汁性胆管炎)诊断和治疗共识(2015)[J]. 临床肝胆病杂志, 2015, 31(12): 1980-1988. DOI: 10.3969/j.issn.1001-5256.2015.12.004. [11] CZAJA AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis[J]. Hepatology, 2008, 48(5): 1540-1548. DOI: 10.1002/hep.22513. [12] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and management of primary sclerosing cholangitis(2015)[J]. J Clin Hepatol, 2016, 32(1): 23-31. DOI: 10.3969/j.issn.1001-5256.2016.01.003.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 原发性硬化性胆管炎诊断和治疗专家共识(2015)[J]. 临床肝胆病杂志, 2016, 32(1): 23-31. DOI: 10.3969/j.issn.1001-5256.2016.01.003. [13] PRUENSTER M, KURZ AR, CHUNG KJ, et al. Extracellular MRP8/14 is a regulator of β2 integrin-dependent neutrophil slow rolling and adhesion[J]. Nat Commun, 2015, 6: 6915. DOI: 10.1038/ncomms7915. [14] O'NEIL LJ, BARRERA-VARGAS A, SANDOVAL-HEGLUND D, et al. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis[J]. Sci Adv, 2020, 6(44): eabd2688. DOI: 10.1126/sciadv.abd2688. [15] PARK SY, SHRESTHA S, YOUN YJ, et al. Autophagy primes neutrophils for neutrophil extracellular trap formation during sepsis[J]. Am J Respir Crit Care Med, 2017, 196(5): 577-589. DOI: 10.1164/rccm.201603-0596OC. [16] MISTRY P, CARMONA-RIVERA C, OMBRELLO AK, et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome[J]. Ann Rheum Dis, 2018, 77(12): 1825-1833. DOI: 10.1136/annrheumdis-2018-213746. [17] SUROLIA R, LI FJ, WANG Z, et al. NETosis in the pathogenesis of acute lung injury following cutaneous chemical burns[J]. JCI Insight, 2021, 6(10): e147564. DOI: 10.1172/jci.insight.147564. [18] EKSTEEN B, AFFORD SC, WIGMORE SJ, et al. Immune-mediated liver injury[J]. Semin Liver Dis, 2007, 27(4): 351-366. DOI: 10.1055/s-2007-991512. [19] TAKEUCHI M, VIDIGAL PT, GUERRA MT, et al. Neutrophils interact with cholangiocytes to cause cholestatic changes in alcoholic hepatitis[J]. Gut, 2021, 70(2): 342-356. DOI: 10.1136/gutjnl-2020-322540. [20] BARRERA-VARGAS A, GÓMEZ-MARTÍN D, CARMONA-RIVERA C, et al. Differential ubiquitination in NETs regulates macrophage responses in systemic lupus erythematosus[J]. Ann Rheum Dis, 2018, 77(6): 944-950. DOI: 10.1136/annrheumdis-2017-212617. [21] BERTELLI R, SCHENA F, ANTONINI F, et al. Neutrophil extracellular traps in systemic lupus erythematosus stimulate IgG2 production from B lymphocytes[J]. Front Med (Lausanne), 2021, 8: 635436. DOI: 10.3389/fmed.2021.635436. [22] LI T, WANG C, LIU Y, et al. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease[J]. J Crohns Colitis, 2020, 14(2): 240-253. DOI: 10.1093/ecco-jcc/jjz132. [23] PERDOMO J, LEUNG H, AHMADI Z, et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia[J]. Nat Commun, 2019, 10(1): 1322. DOI: 10.1038/s41467-019-09160-7. [24] LE JONCOUR A, MARTOS R, LOYAU S, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet's disease[J]. Ann Rheum Dis, 2019, 78(9): 1274-1282. DOI: 10.1136/annrheumdis-2018-214335. [25] LOOD C, BLANCO LP, PURMALEK MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease[J]. Nat Med, 2016, 22(2): 146-153. DOI: 10.1038/nm.4027. [26] HILSCHER MB, SHAH VH. Neutrophil extracellular traps and liver disease[J]. Semin Liver Dis, 2020, 40(2): 171-179. DOI: 10.1055/s-0039-3399562. -



 PDF下载 ( 3548 KB)
PDF下载 ( 3548 KB)


 下载:
下载: