尽管HBsAg转阴已被定义为慢性乙肝治疗的理想终点,但是通过长期的核苷(酸)类似物(NUC)治疗很难实现。这项NEW SWITCH研究旨在评估NUC经治的HBeAg阳性慢性乙肝患者通过PEG-IFN α-2a(40KD)转换治疗实现HBsAg转阴的可能性。
研究方法
NEW SWITCH是一项多中心、随机、非盲的研究。研究纳入经过NUC治疗1-3年实现部分应答(应答定义为HBVDNA<200IU/ml和HBeAg转阴)的HBeAg阳性CHB患者。所有受试者按照1:1被随机分配接受PEG-IFNα-2a治疗48周或者96周(前12周联合NUC治疗),PEG-IFNα-2a治疗停止后随访监测48周。治疗48周时,HBsAg转阴率是本次中期分析的主要终点。
研究结果
共有303例患者被随机分组并纳入ITT人群。平均年龄为34.2岁。基线的qHBsAg和HBeAg水平分别为3.105(±0.7844)logIU/ml,-0.654(±0.1515)logPEIU/ml。
经过48周治疗后,HBsAg转阴率和血清转换率达到16.2%和12.5%。实现HBsAg≤1000 IU/ml,≤100 IU/ml,≤10IU/ml的患者分别为65.7%,46.5%和25.7%。共有58.7%的患者实现HBeAg血清学转换。在整个研究中,91.7%的受试者保持HBV DNA抑制,59.1%的受试者达到ALT复常(定义为ALT≤ULN)。
基线时qHBsAg水平低(<1500 IU/ml)的患者,在分析末时实现了更高的HBsAg转阴率和更高的HBsAg<10 IU/ml的比率(图1)。
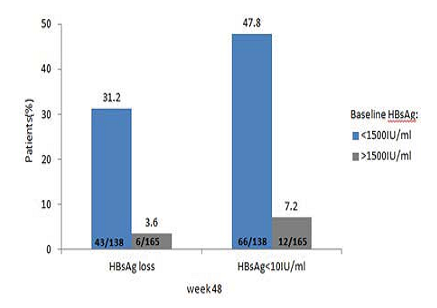
图1. 基线qHBsAg水平的分层分析结果
安全性类似于以前的研究。多数不良事件为轻度至中度,而SAE的发生率为3.3%。
结论
实现部分应答的HBeAg阳性CHB患者很可能从PEG-IFN的转换治疗中获得高的HBsAg清除率,特别是那些基线qHBsAg水平低的患者。NEW SWITCH的最终结果值得关注,以期进一步明确更好的治疗策略和更能从PEG-IFN治疗中获益的人群。
英文原文
A multi-center randomized study on the efficacy and safety of switching to peginterferonα-2a (40 KD)for 48 or 96 weeks in HBeAg positive CHB patients with a prior NUC history for 1 to 3 years: an interim analysis of NEW SWITCH study.
Background and aim: Although HBsAg loss has been defined as the ideal endpoint of CHB treatment, it is difficult to achieve with long-term NLIC therapy. The aim of the NEW SWITCH study is to evaluate the possibility of NIJC-experienced HBeAg positive CHB patients to achieve HBsAg loss with PEG-IFNa-2a (40KD) therapy.
Method: NEW SWITCH is a multi-centered randomized open-labelled study. HBeAg positive CHB patients who achieved partial responses (defined as HBV DNAQCClLl/ml and HBeAg loss) with a prior NIJC history for 1-3 years were included. All participants were switched to PEG-IFNa-2a treatment for either 48 or g6 weeks (with the first 12 weeks overlapping NLIC therapy) at a randomization ratio of , and followed-up for 48 weeks after the discontinuation of PEG-IFNa-2a. HBsAg loss rate at 48 weeks since randomization was the primary endpoint in this interim analysis.
Results: A total of 303 patients were randomized and included in the ITT population. The mean age was 34.2 yrs. Baseline levels of qHBsAg and HBeAg were 3.105 (+0.7844) loglU,'mI and -0.654 (+0.1515) log PEIU/mL, respectively.
After 4B weeks treatment, the HBsAg loss and seroconversion rate reached 16.2% and 12.5%. Patients who achieved HBsAg≤100 IU/ml, SIOOIU,'mI and SICIU/ml were 65.7%, 46.5% and 25.7%, respectively. A total of 58.7% patients had HBeAg seroconversion. Throughout the study, 91.7% participants maintained HBV DNA suppression and 59.1 % achieved ALT normalization (defined as ALTSULN).
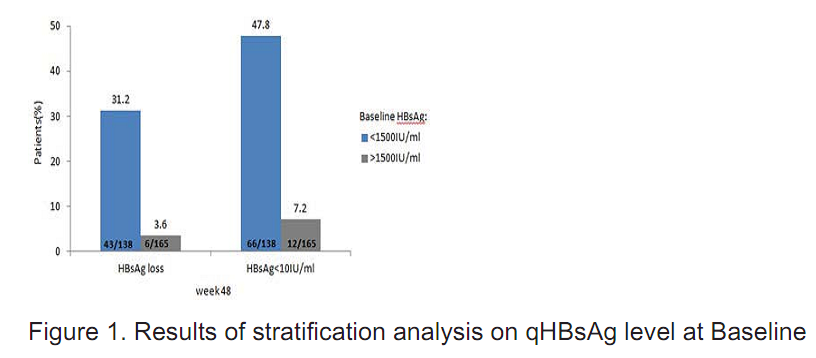
Patients with lower qHBsAg kvel at baseline (K15001Ll/ml) achieved higher HBsAg loss rate and higher rate of HBsAge1ClU/rnl at the end of the analysis (Figure 1).
The safety profile was similar to previous studies. The majority of adverse events were mild to moderate while the incidence of SAE was 3.3%.
Conclusion: HBeAg positive CHB patients who had partial responses are likely to achieve high HBsAg clearance rate by switching to PEG-IFN therapy, especially for those who have lower qHBsAg evel at baseline. Final results of NEW SWITCH are worth attention to further identify the better treatment strategy and the patient population who may benefit more from PEG-IFN therapy.


































